/ CompletedNot Applicable 布瑞哌唑口溶膜(2mg)在中国健康志愿者中空腹/餐后状态下的人体生物等效性试验
[Translation] Human bioequivalence study of brepazine orodispersible film (2 mg) in Chinese healthy volunteers under fasting/fed conditions
主要目的:按有关生物等效性试验的规定,选择Otsuka Pharmaceutical Netherlands B.V.为持证商的布瑞哌唑片(RXULTI®,规格:2mg)为参比制剂,对深圳市宇健生物医药有限公司提供的受试制剂布瑞哌唑口溶膜(规格:2mg)进行空腹和餐后给药条件下的人体生物等效性试验,比较受试制剂与参比制剂的药代动力学特征,评价两种制剂在空腹或餐后给药条件下的生物等效性。
次要目的:评价布瑞哌唑口溶膜的适口性(包括味道、气味、余味)、砂砾感、粘附性和刺激性;评价单剂量口服受试制剂布瑞哌唑口溶膜(规格:2mg)及参比制剂布瑞哌唑片(规格:2mg)在中国健康受试者中的安全性。
[Translation] Primary objective: According to the relevant provisions of bioequivalence tests, brepirazole tablets (RXULTI®, specification: 2 mg) of Otsuka Pharmaceutical Netherlands B.V. were selected as the reference preparation. The test preparation brepirazole orodispersible film (specification: 2 mg) provided by Shenzhen Yujian Biopharmaceutical Co., Ltd. was subjected to human bioequivalence tests under fasting and postprandial administration conditions, and the pharmacokinetic characteristics of the test preparation and the reference preparation were compared to evaluate the bioequivalence of the two preparations under fasting or postprandial administration conditions.
Secondary objectives: To evaluate the palatability (including taste, odor, aftertaste), grittiness, adhesion and irritation of brepirazole orodispersible film; to evaluate the safety of single-dose oral administration of the test preparation brepirazole orodispersible film (specification: 2 mg) and the reference preparation brepirazole tablets (specification: 2 mg) in healthy Chinese subjects.
高脂饮食对健康受试者口服NF2105胶囊的药代动力学以及QT/QTc间期影响的对比研究
[Translation] Comparative study on the effects of high-fat diet on the pharmacokinetics and QT/QTc interval of oral NF2105 capsules in healthy subjects
(1)评价高脂饮食对健康受试者单次口服NF2105胶囊的药代动力学影响;
(2)评价NF2105胶囊(餐前/餐后)及尼洛替尼(400mg)胶囊空腹给药后对中国健康受试者QT/QTc间期的影响;
(3)初步评价健康受试者单次空腹口服NF2105胶囊与尼洛替尼胶囊的相对生物利用度;
(4)评价健康受试者单次口服NF2105胶囊(空腹或餐后)及尼洛替尼胶囊(空腹)的PK特征以及PK/PD(QT/QTc间期)的特性;
(5)评价健康受试者口服NF2105胶囊或尼洛替尼胶囊的安全性。
[Translation] (1) To evaluate the effect of a high-fat diet on the pharmacokinetic response of a single oral administration of NF2105 capsules to healthy subjects;
(2) To evaluate the effect of NF2105 capsules (before or after meals) and nilotinib (400 mg) capsules on the QT/QTc interval after fasting administration to healthy Chinese subjects;
(3) To preliminarily evaluate the relative bioavailability of NF2105 capsules and nilotinib capsules after a single oral administration to healthy subjects on an empty stomach;
(4) To evaluate the PK characteristics and PK/PD (QT/QTc interval) of a single oral administration of NF2105 capsules (fasting or after meals) and nilotinib capsules (fasting) to healthy subjects;
(5) To evaluate the safety of oral administration of NF2105 capsules or nilotinib capsules to healthy subjects.
NF2105胶囊及尼洛替尼胶囊在健康受试者的剂量比例关系研究
[Translation] Study on the dose ratio relationship between NF2105 capsules and nilotinib capsules in healthy subjects
主要目的:考察健康受试者单次空腹口服不同剂量NF2105的PK特征及剂量比例关系;比较健康受试者单次空腹口服NF2105及尼洛替尼胶囊的PK特征,探索两者具有相似暴露水平的剂量。次要目的:评价健康受试者单次空腹口服不同剂量NF2105的安全性和耐受性;评价NF2105及尼洛替尼胶囊对健康受试者QT/QTc间期的影响。
[Translation] Primary objective: To investigate the PK characteristics and dose-proportion relationship of NF2105 in healthy subjects after single oral administration of different doses on an empty stomach; To compare the PK characteristics of NF2105 and nilotinib capsules in healthy subjects after single oral administration on an empty stomach, and to explore the doses with similar exposure levels. Secondary objective: To evaluate the safety and tolerability of NF2105 in healthy subjects after single oral administration of different doses on an empty stomach; To evaluate the effects of NF2105 and nilotinib capsules on the QT/QTc interval in healthy subjects.
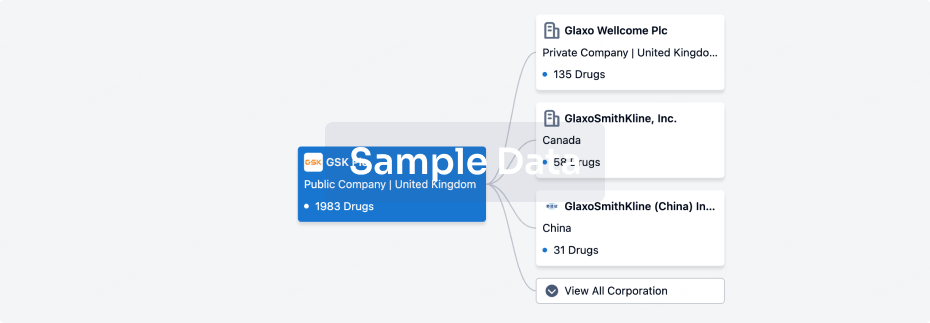
100 Clinical Results associated with Shenzhen Taili Biomedicine Co., Ltd.
0 Patents (Medical) associated with Shenzhen Taili Biomedicine Co., Ltd.
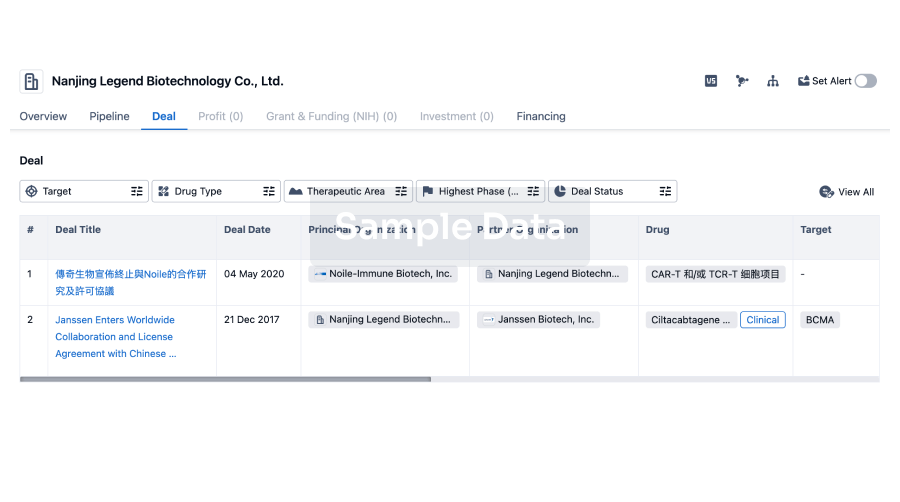
100 Deals associated with Shenzhen Taili Biomedicine Co., Ltd.
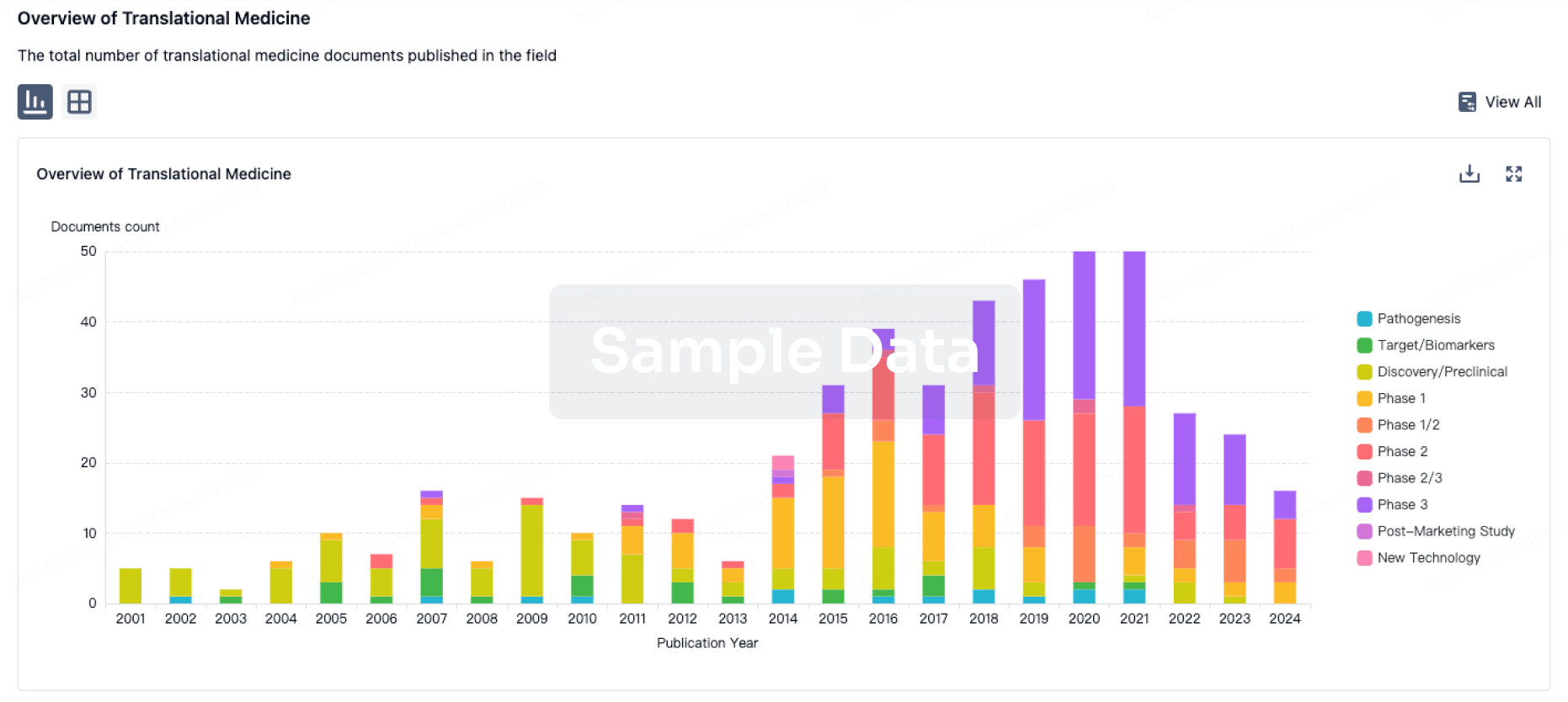
100 Translational Medicine associated with Shenzhen Taili Biomedicine Co., Ltd.