Request Demo
What is Avacincaptad pegol used for?
17 June 2024
Avacincaptad pegol is a promising therapeutic agent emerging in the landscape of ophthalmology. Known by its trade name, Zimura, Avacincaptad pegol is an innovative complement inhibitor targeting the complement protein C5. Research and development of this drug have been spearheaded by Iveric Bio, a biopharmaceutical company dedicated to discovering and developing novel treatments for retinal diseases. As a type of nucleic acid-based aptamer, Avacincaptad pegol is primarily indicated for the treatment of geographic atrophy (GA), a severe form of age-related macular degeneration (AMD). With AMD being a leading cause of vision loss among the elderly, the advent of Avacincaptad pegol marks a significant milestone. Currently, the drug is in advanced stages of clinical trials and has shown promising results in reducing the progression of GA.
Avacincaptad pegol operates through a targeted mechanism of action centered on the inhibition of the complement system, specifically the C5 protein. The complement system is a crucial component of the immune system involved in inflammation and defense against pathogens. However, dysregulation of this system has been implicated in the pathogenesis of several diseases, including AMD. By binding to C5, Avacincaptad pegol prevents its cleavage into C5a and C5b, thereby hindering the formation of the membrane attack complex (MAC). This action mitigates inflammation and cellular damage, which are pivotal factors in the progression of geographic atrophy. Essentially, Avacincaptad pegol curbs the cascade of immune reactions that lead to the degeneration of retinal cells, offering a targeted approach to preserving vision in patients with AMD.
Administering Avacincaptad pegol involves intravitreal injections, a common delivery method for retinal therapeutics. Intravitreal injections are administered directly into the vitreous humor of the eye, ensuring the drug reaches its specific target in the retina. This localized delivery method maximizes the drug's efficacy while minimizing systemic exposure and potential side effects. Avacincaptad pegol is typically injected once monthly, although the exact dosing schedule may vary based on the specific needs and responses of individual patients. The onset of action for Avacincaptad pegol can be observed relatively quickly, with some studies indicating improvements in retinal health markers within a few weeks of initiation. Regular monitoring by an ophthalmologist is essential to assess the therapeutic response and adjust the treatment regimen as necessary.
Like all medications, Avacincaptad pegol is associated with potential side effects and contraindications. The most common adverse effects reported in clinical trials include eye pain, discomfort, and increased intraocular pressure following the injection. These side effects are generally mild to moderate and tend to resolve without significant intervention. However, serious complications such as endophthalmitis, retinal detachment, and increased intraocular inflammation, although rare, have been documented. Patients with a history of ocular or systemic infections, or those who are hypersensitive to any component of the drug, should not receive Avacincaptad pegol. Additionally, sterile techniques during administration are crucial to minimize the risk of infection and other complications. Close monitoring for any adverse reactions following the injection is imperative to ensure patient safety and effective management of side effects.
The interaction of Avacincaptad pegol with other drugs is an important consideration for clinicians. Due to its localized delivery and targeted mechanism of action, systemic drug interactions are less likely compared to systemic medications. However, caution is advised when Avacincaptad pegol is administered concurrently with other intravitreal therapeutics, particularly those with similar mechanisms of action, such as other complement inhibitors or anti-VEGF agents, which are commonly used in the treatment of retinal diseases. Combining these treatments may increase the risk of compounded side effects, such as increased intraocular pressure or inflammation. A comprehensive review of a patient's medication regimen is essential to identify any potential interactions and to devise an appropriate treatment plan. Ongoing research will continue to elucidate the full spectrum of drug interactions and optimize the safety profile of Avacincaptad pegol in clinical practice.
In conclusion, Avacincaptad pegol represents a significant advancement in the treatment of geographic atrophy secondary to age-related macular degeneration. Its targeted inhibition of the complement protein C5 offers a novel approach to mitigating retinal degeneration and preserving vision. With its promising clinical trial outcomes, Avacincaptad pegol holds the potential to become a cornerstone therapy in the management of AMD. As with any medical treatment, careful consideration of administration methods, monitoring for side effects, and an understanding of potential drug interactions are essential to ensuring the optimal therapeutic outcomes for patients. As research progresses, the medical community eagerly anticipates the further development and potential regulatory approval of this groundbreaking drug.
Avacincaptad pegol operates through a targeted mechanism of action centered on the inhibition of the complement system, specifically the C5 protein. The complement system is a crucial component of the immune system involved in inflammation and defense against pathogens. However, dysregulation of this system has been implicated in the pathogenesis of several diseases, including AMD. By binding to C5, Avacincaptad pegol prevents its cleavage into C5a and C5b, thereby hindering the formation of the membrane attack complex (MAC). This action mitigates inflammation and cellular damage, which are pivotal factors in the progression of geographic atrophy. Essentially, Avacincaptad pegol curbs the cascade of immune reactions that lead to the degeneration of retinal cells, offering a targeted approach to preserving vision in patients with AMD.
Administering Avacincaptad pegol involves intravitreal injections, a common delivery method for retinal therapeutics. Intravitreal injections are administered directly into the vitreous humor of the eye, ensuring the drug reaches its specific target in the retina. This localized delivery method maximizes the drug's efficacy while minimizing systemic exposure and potential side effects. Avacincaptad pegol is typically injected once monthly, although the exact dosing schedule may vary based on the specific needs and responses of individual patients. The onset of action for Avacincaptad pegol can be observed relatively quickly, with some studies indicating improvements in retinal health markers within a few weeks of initiation. Regular monitoring by an ophthalmologist is essential to assess the therapeutic response and adjust the treatment regimen as necessary.
Like all medications, Avacincaptad pegol is associated with potential side effects and contraindications. The most common adverse effects reported in clinical trials include eye pain, discomfort, and increased intraocular pressure following the injection. These side effects are generally mild to moderate and tend to resolve without significant intervention. However, serious complications such as endophthalmitis, retinal detachment, and increased intraocular inflammation, although rare, have been documented. Patients with a history of ocular or systemic infections, or those who are hypersensitive to any component of the drug, should not receive Avacincaptad pegol. Additionally, sterile techniques during administration are crucial to minimize the risk of infection and other complications. Close monitoring for any adverse reactions following the injection is imperative to ensure patient safety and effective management of side effects.
The interaction of Avacincaptad pegol with other drugs is an important consideration for clinicians. Due to its localized delivery and targeted mechanism of action, systemic drug interactions are less likely compared to systemic medications. However, caution is advised when Avacincaptad pegol is administered concurrently with other intravitreal therapeutics, particularly those with similar mechanisms of action, such as other complement inhibitors or anti-VEGF agents, which are commonly used in the treatment of retinal diseases. Combining these treatments may increase the risk of compounded side effects, such as increased intraocular pressure or inflammation. A comprehensive review of a patient's medication regimen is essential to identify any potential interactions and to devise an appropriate treatment plan. Ongoing research will continue to elucidate the full spectrum of drug interactions and optimize the safety profile of Avacincaptad pegol in clinical practice.
In conclusion, Avacincaptad pegol represents a significant advancement in the treatment of geographic atrophy secondary to age-related macular degeneration. Its targeted inhibition of the complement protein C5 offers a novel approach to mitigating retinal degeneration and preserving vision. With its promising clinical trial outcomes, Avacincaptad pegol holds the potential to become a cornerstone therapy in the management of AMD. As with any medical treatment, careful consideration of administration methods, monitoring for side effects, and an understanding of potential drug interactions are essential to ensuring the optimal therapeutic outcomes for patients. As research progresses, the medical community eagerly anticipates the further development and potential regulatory approval of this groundbreaking drug.
How to obtain the latest development progress of all drugs?
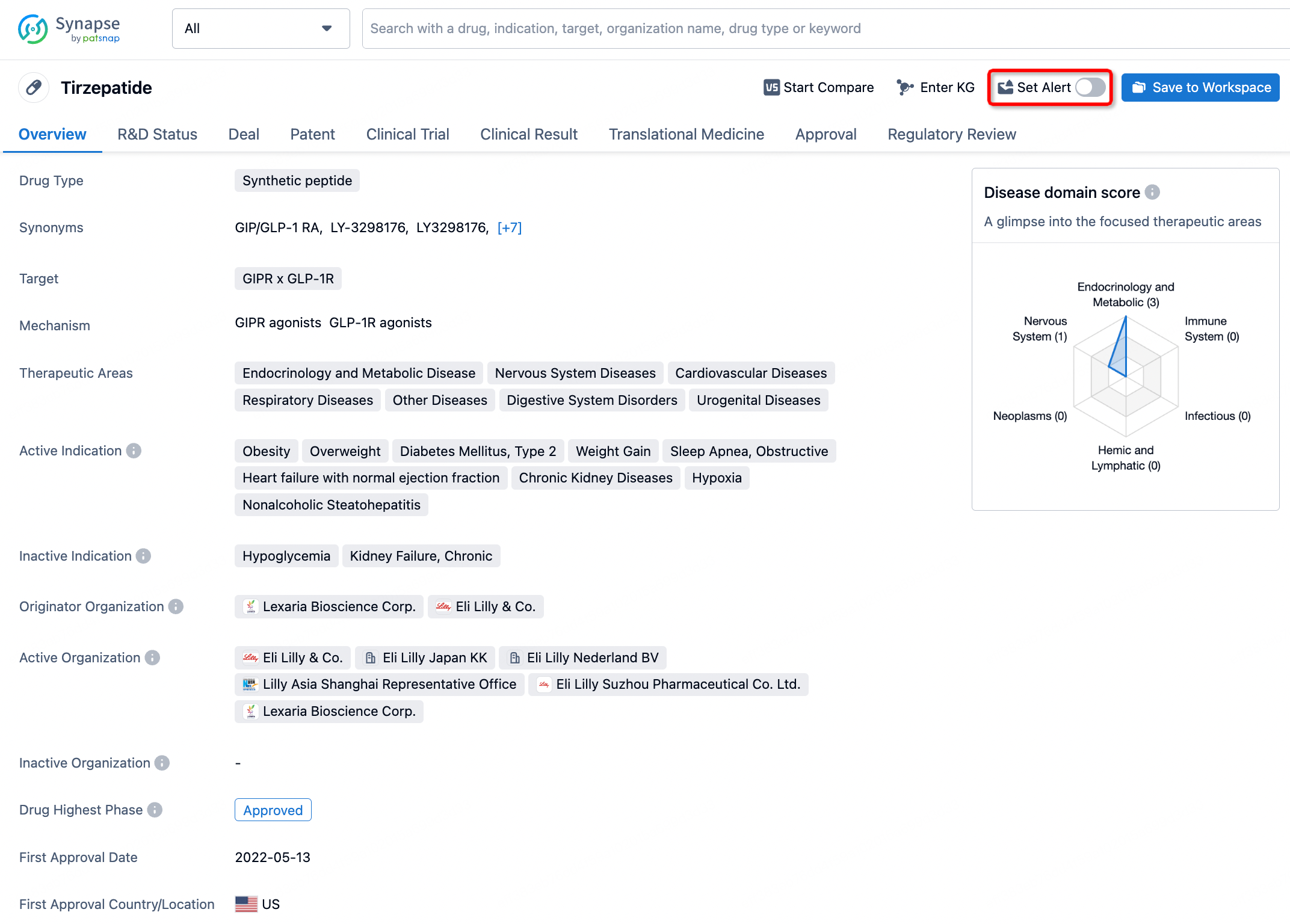
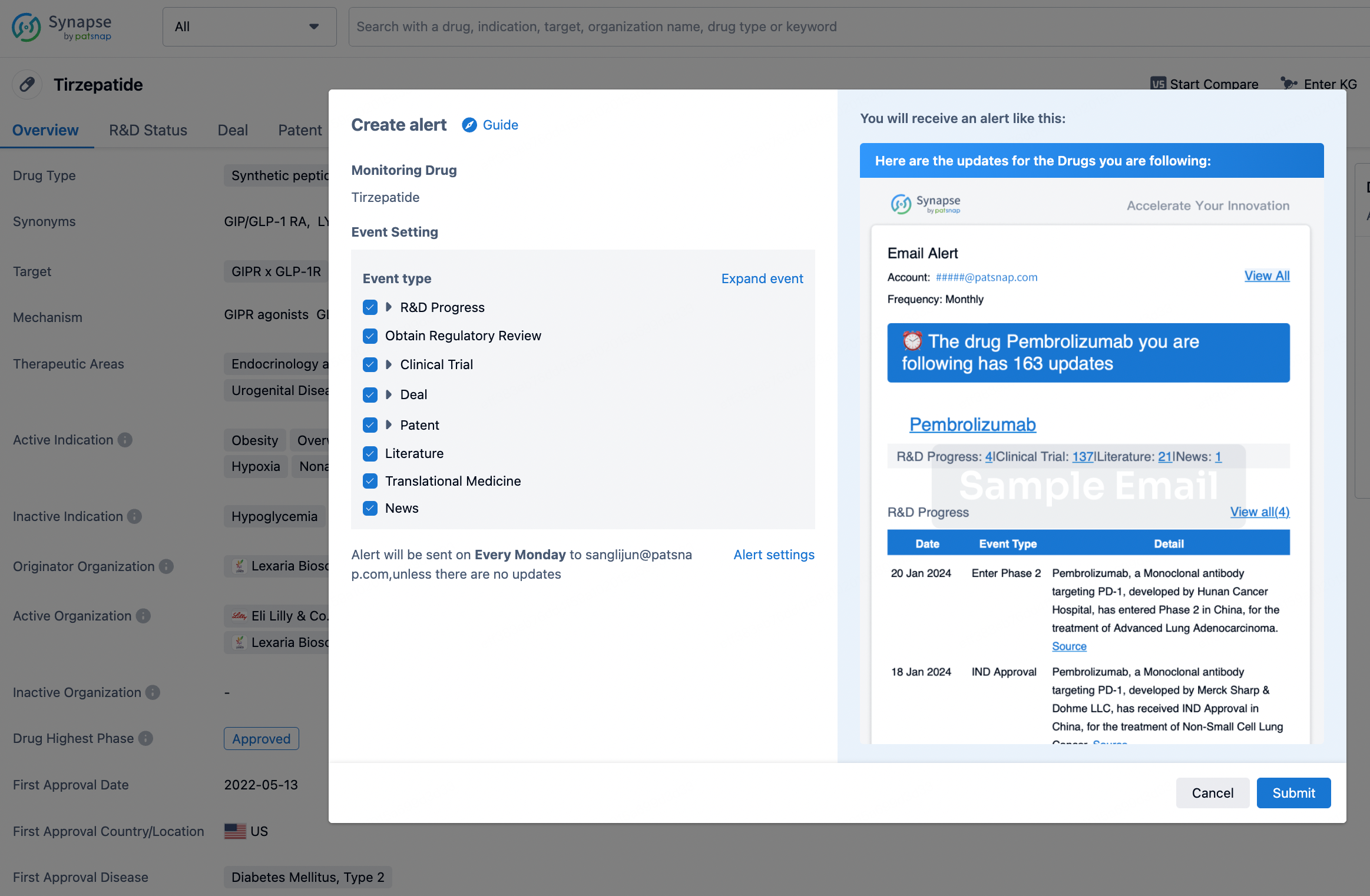
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.