Request Demo
What is Inebilizumab-cdon used for?
14 June 2024
Inebilizumab-cdon is an innovative monoclonal antibody that has garnered significant attention in recent years for its potential therapeutic benefits. Known by its trade name Uplizna, this drug has been developed as a treatment for neuromyelitis optica spectrum disorder (NMOSD), a rare and debilitating autoimmune condition that primarily affects the optic nerves and spinal cord. The drug targets CD19, a protein found on the surface of B cells, which are implicated in the pathogenesis of NMOSD. Research institutions, including Viela Bio (now part of Horizon Therapeutics), have been at the forefront of developing and studying Inebilizumab-cdon. This monoclonal antibody is designed to deplete B cells, thereby reducing the inflammatory processes that contribute to NMOSD. Since receiving FDA approval in June 2020, Uplizna has offered a new line of defense for patients suffering from this chronic condition, particularly for those who have not responded well to other treatments.
Inebilizumab-cdon exerts its therapeutic effects through a targeted mechanism aimed at B cells, a type of white blood cell that plays a critical role in the immune response. Specifically, Inebilizumab-cdon binds to the CD19 antigen, which is expressed on the surface of B cells. By attaching to CD19, the drug induces the depletion of these cells through a process known as antibody-dependent cellular cytotoxicity (ADCC) as well as complement-dependent cytotoxicity (CDC). This depletion of B cells is crucial because these cells are involved in the production of autoantibodies that attack the body's own tissues in NMOSD. By reducing the number of B cells, Inebilizumab-cdon helps to mitigate the autoimmune response, thereby decreasing inflammation and preventing further damage to the optic nerves and spinal cord. This targeted approach not only provides symptom relief but also helps to prevent relapses, offering a significant improvement in the quality of life for patients with NMOSD.
The administration of Inebilizumab-cdon is a straightforward process, typically carried out in a clinical setting under the supervision of healthcare professionals. The drug is administered via intravenous infusion, ensuring that it is delivered directly into the bloodstream for maximum efficacy. The infusion process generally takes about 90 minutes to complete. The initial treatment regimen involves two infusions spaced two weeks apart, which helps to rapidly deplete the B cells and achieve a quick onset of action. Subsequent maintenance doses are administered every six months to sustain the therapeutic effects and continue B cell depletion. Patients usually start to experience symptom relief within a few weeks after the initial infusions, although the timing can vary depending on individual factors such as disease severity and overall health. It is essential for patients to adhere to the prescribed infusion schedule to maintain the drug's efficacy and prevent relapses.
Like all medications, Inebilizumab-cdon is associated with a range of potential side effects, which patients and healthcare providers should be aware of. Common side effects include infusion-related reactions such as headache, nausea, fatigue, and fever, which typically occur during or shortly after the infusion process. These reactions are usually mild to moderate in severity and can often be managed with premedication and supportive care. More serious side effects, although less common, can include infections, as the depletion of B cells may compromise the immune system's ability to fight off pathogens. Therefore, patients should be closely monitored for signs of infection, and preventive measures, such as vaccinations, may be recommended prior to initiating treatment.
There are also specific contraindications for the use of Inebilizumab-cdon. Patients with a known hypersensitivity to the drug or any of its components should not receive it. Additionally, those with active infections should defer treatment until the infection is adequately controlled. It is also advisable to screen for hepatitis B and tuberculosis before starting therapy, as reactivation of these infections can occur in immunocompromised states.
The use of Inebilizumab-cdon can potentially be influenced by interactions with other medications, making it essential for patients to inform their healthcare providers about all the drugs they are taking, including over-the-counter medications, supplements, and herbal products. Immunosuppressive drugs, such as corticosteroids, other monoclonal antibodies, and certain chemotherapeutic agents, may enhance the immunosuppressive effects of Inebilizumab-cdon, increasing the risk of infections. On the other hand, drugs that affect the immune system or the metabolism of monoclonal antibodies can potentially alter the efficacy and safety profile of Inebilizumab-cdon.
Moreover, live vaccines should be avoided during treatment with Inebilizumab-cdon due to the risk of infection. It is recommended that any necessary vaccinations be administered at least four weeks prior to starting therapy. Additionally, patients should be monitored for any signs of increased or unusual side effects when starting or stopping concomitant medications.
In conclusion, Inebilizumab-cdon represents a significant advancement in the treatment of neuromyelitis optica spectrum disorder, offering hope to patients suffering from this challenging condition. Its targeted mechanism of action, focusing on the depletion of B cells, helps to reduce inflammation and prevent relapses, thereby improving patient outcomes. However, as with any medication, it is crucial to be aware of potential side effects and drug interactions to ensure safe and effective use. Through ongoing research and clinical experience, our understanding of Inebilizumab-cdon continues to evolve, paving the way for improved therapies and management strategies for NMOSD.
Inebilizumab-cdon exerts its therapeutic effects through a targeted mechanism aimed at B cells, a type of white blood cell that plays a critical role in the immune response. Specifically, Inebilizumab-cdon binds to the CD19 antigen, which is expressed on the surface of B cells. By attaching to CD19, the drug induces the depletion of these cells through a process known as antibody-dependent cellular cytotoxicity (ADCC) as well as complement-dependent cytotoxicity (CDC). This depletion of B cells is crucial because these cells are involved in the production of autoantibodies that attack the body's own tissues in NMOSD. By reducing the number of B cells, Inebilizumab-cdon helps to mitigate the autoimmune response, thereby decreasing inflammation and preventing further damage to the optic nerves and spinal cord. This targeted approach not only provides symptom relief but also helps to prevent relapses, offering a significant improvement in the quality of life for patients with NMOSD.
The administration of Inebilizumab-cdon is a straightforward process, typically carried out in a clinical setting under the supervision of healthcare professionals. The drug is administered via intravenous infusion, ensuring that it is delivered directly into the bloodstream for maximum efficacy. The infusion process generally takes about 90 minutes to complete. The initial treatment regimen involves two infusions spaced two weeks apart, which helps to rapidly deplete the B cells and achieve a quick onset of action. Subsequent maintenance doses are administered every six months to sustain the therapeutic effects and continue B cell depletion. Patients usually start to experience symptom relief within a few weeks after the initial infusions, although the timing can vary depending on individual factors such as disease severity and overall health. It is essential for patients to adhere to the prescribed infusion schedule to maintain the drug's efficacy and prevent relapses.
Like all medications, Inebilizumab-cdon is associated with a range of potential side effects, which patients and healthcare providers should be aware of. Common side effects include infusion-related reactions such as headache, nausea, fatigue, and fever, which typically occur during or shortly after the infusion process. These reactions are usually mild to moderate in severity and can often be managed with premedication and supportive care. More serious side effects, although less common, can include infections, as the depletion of B cells may compromise the immune system's ability to fight off pathogens. Therefore, patients should be closely monitored for signs of infection, and preventive measures, such as vaccinations, may be recommended prior to initiating treatment.
There are also specific contraindications for the use of Inebilizumab-cdon. Patients with a known hypersensitivity to the drug or any of its components should not receive it. Additionally, those with active infections should defer treatment until the infection is adequately controlled. It is also advisable to screen for hepatitis B and tuberculosis before starting therapy, as reactivation of these infections can occur in immunocompromised states.
The use of Inebilizumab-cdon can potentially be influenced by interactions with other medications, making it essential for patients to inform their healthcare providers about all the drugs they are taking, including over-the-counter medications, supplements, and herbal products. Immunosuppressive drugs, such as corticosteroids, other monoclonal antibodies, and certain chemotherapeutic agents, may enhance the immunosuppressive effects of Inebilizumab-cdon, increasing the risk of infections. On the other hand, drugs that affect the immune system or the metabolism of monoclonal antibodies can potentially alter the efficacy and safety profile of Inebilizumab-cdon.
Moreover, live vaccines should be avoided during treatment with Inebilizumab-cdon due to the risk of infection. It is recommended that any necessary vaccinations be administered at least four weeks prior to starting therapy. Additionally, patients should be monitored for any signs of increased or unusual side effects when starting or stopping concomitant medications.
In conclusion, Inebilizumab-cdon represents a significant advancement in the treatment of neuromyelitis optica spectrum disorder, offering hope to patients suffering from this challenging condition. Its targeted mechanism of action, focusing on the depletion of B cells, helps to reduce inflammation and prevent relapses, thereby improving patient outcomes. However, as with any medication, it is crucial to be aware of potential side effects and drug interactions to ensure safe and effective use. Through ongoing research and clinical experience, our understanding of Inebilizumab-cdon continues to evolve, paving the way for improved therapies and management strategies for NMOSD.
How to obtain the latest development progress of all drugs?
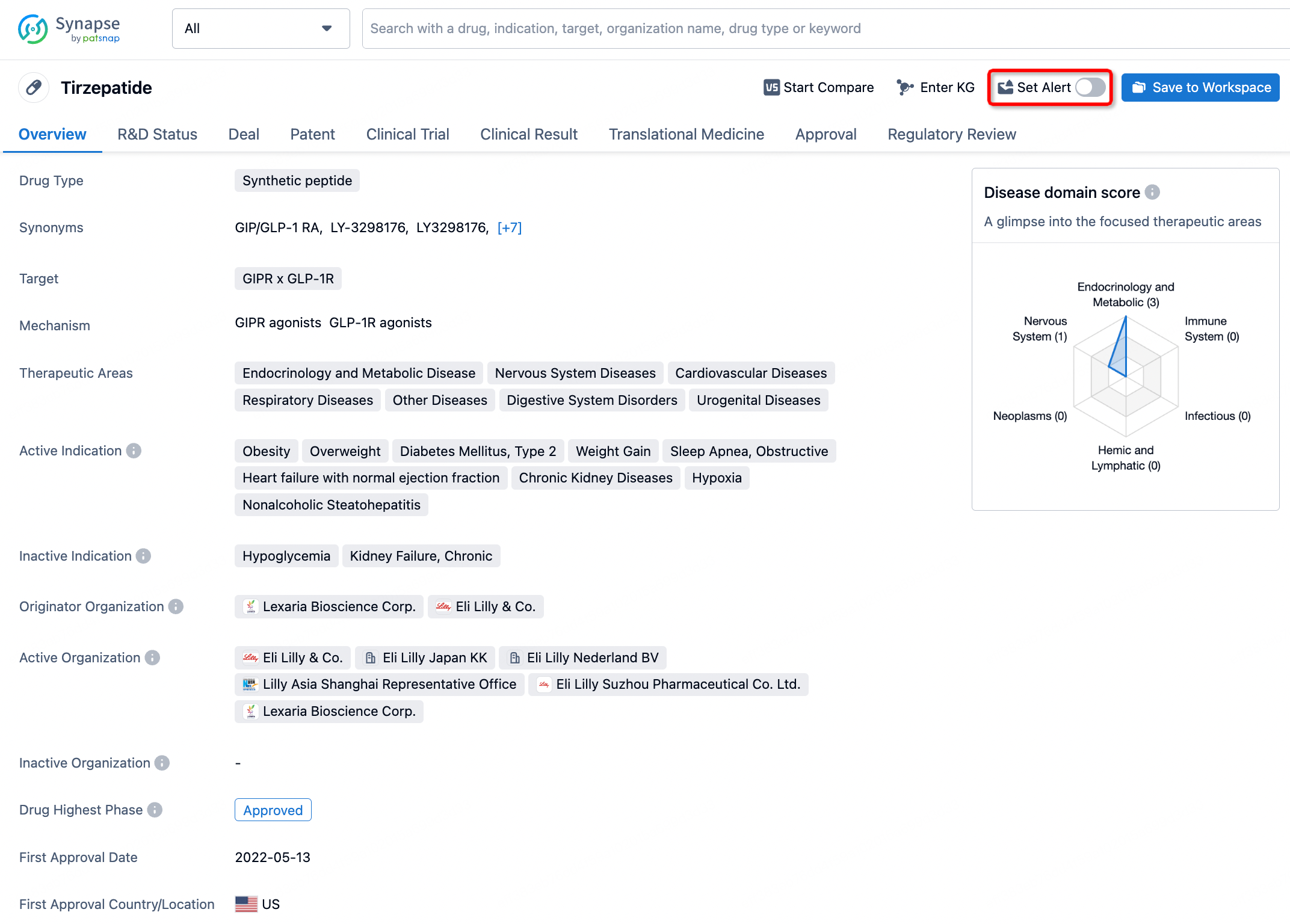
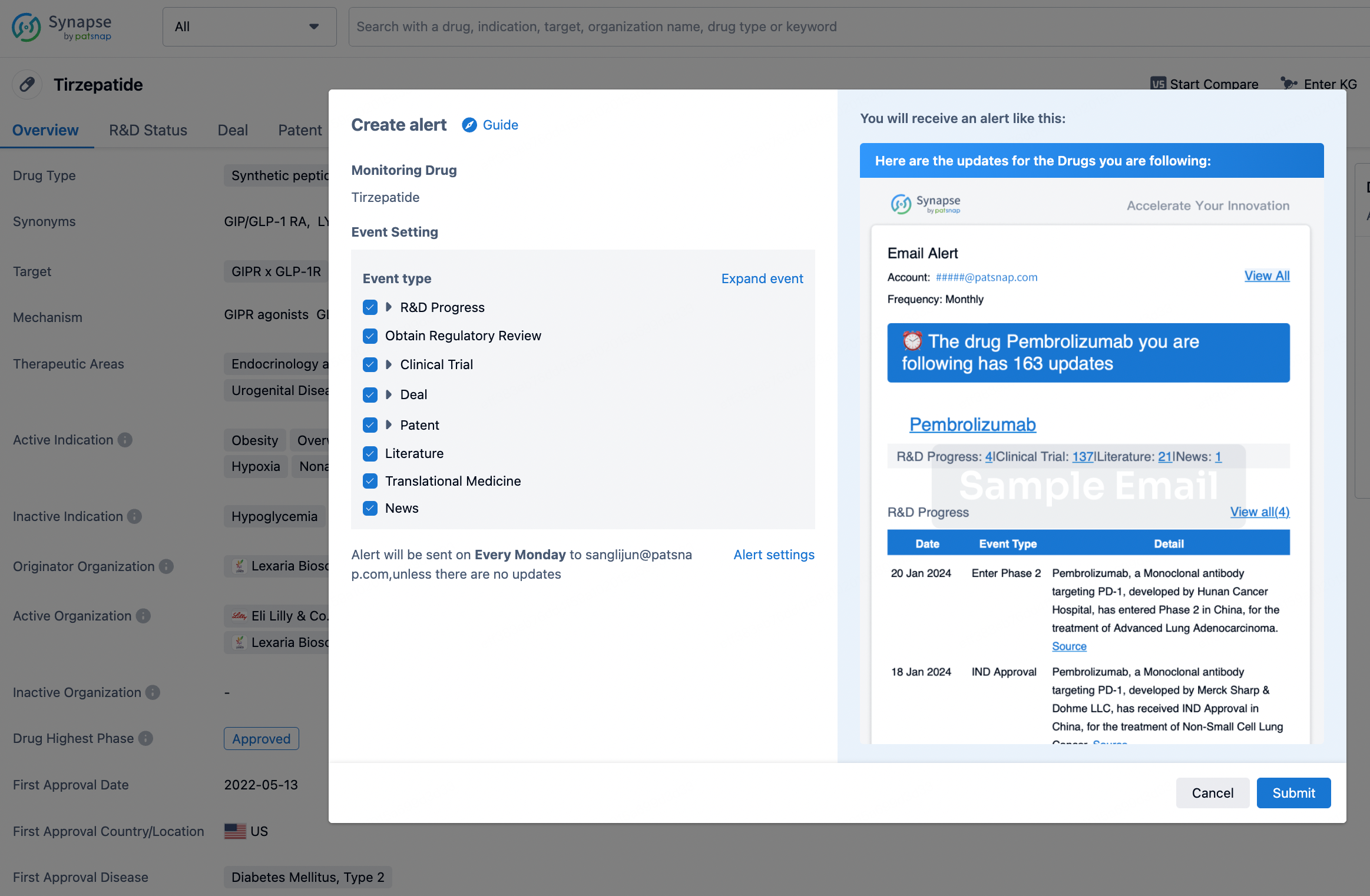
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.