Request Demo
What is the mechanism of Silver Nitrate?
18 July 2024
Silver nitrate, a chemical compound with the formula AgNO3, is an important reagent in various scientific and industrial applications. Understanding its mechanism of action can provide valuable insights into its roles in photography, medicine, and chemical synthesis.
Silver nitrate is highly soluble in water, dissociating into silver ions (Ag⁺) and nitrate ions (NO3⁻). The silver ion is the active component and is responsible for the compound's chemical reactivity and biological activity. One of the significant properties of silver ions is their ability to bind to and disrupt various biological molecules, including proteins, DNA, and cell membranes. This makes silver nitrate an effective antimicrobial agent. When silver ions come into contact with bacterial cells, they can bind to thiol groups in proteins, leading to the denaturation of these proteins and subsequent cell death. This mechanism is exploited in medical applications such as wound dressings, where silver nitrate helps prevent infections.
In the realm of chemical synthesis, silver nitrate is often used as a precursor for the synthesis of other silver compounds, including silver halides, which are vital in photographic processes. When silver nitrate reacts with halide ions (such as chloride, bromide, or iodide), it precipitates as silver halide, which is light-sensitive and forms the basis of traditional photographic films. The reaction is straightforward: AgNO3 + X⁻ → AgX + NO3⁻, where X represents a halide ion.
Silver nitrate also participates in organic synthesis, particularly in the formation of carbon-carbon and carbon-heteroatom bonds. It often acts as an oxidizing agent due to its ability to accept electrons. For example, in the Tollens' test for aldehydes, silver nitrate in ammonia solution forms the silver-ammonia complex [Ag(NH3)2]⁺, which can oxidize aldehydes to carboxylic acids while reducing silver ions to metallic silver, producing a characteristic silver mirror.
In addition to its chemical reactivity, silver nitrate is employed in analytical chemistry for titrations involving halides, cyanides, and thiocyanates. Known as argentometric titrations, these methods rely on the formation of insoluble silver salts to determine the concentration of analytes in solution.
In summary, the mechanism of silver nitrate's action is primarily attributed to the reactivity of silver ions (Ag⁺). These ions participate in a variety of reactions, ranging from antimicrobial activity and photochemical processes to organic and analytical chemistry applications. Silver nitrate's versatility and effectiveness make it a crucial compound in numerous scientific and industrial domains.
Silver nitrate is highly soluble in water, dissociating into silver ions (Ag⁺) and nitrate ions (NO3⁻). The silver ion is the active component and is responsible for the compound's chemical reactivity and biological activity. One of the significant properties of silver ions is their ability to bind to and disrupt various biological molecules, including proteins, DNA, and cell membranes. This makes silver nitrate an effective antimicrobial agent. When silver ions come into contact with bacterial cells, they can bind to thiol groups in proteins, leading to the denaturation of these proteins and subsequent cell death. This mechanism is exploited in medical applications such as wound dressings, where silver nitrate helps prevent infections.
In the realm of chemical synthesis, silver nitrate is often used as a precursor for the synthesis of other silver compounds, including silver halides, which are vital in photographic processes. When silver nitrate reacts with halide ions (such as chloride, bromide, or iodide), it precipitates as silver halide, which is light-sensitive and forms the basis of traditional photographic films. The reaction is straightforward: AgNO3 + X⁻ → AgX + NO3⁻, where X represents a halide ion.
Silver nitrate also participates in organic synthesis, particularly in the formation of carbon-carbon and carbon-heteroatom bonds. It often acts as an oxidizing agent due to its ability to accept electrons. For example, in the Tollens' test for aldehydes, silver nitrate in ammonia solution forms the silver-ammonia complex [Ag(NH3)2]⁺, which can oxidize aldehydes to carboxylic acids while reducing silver ions to metallic silver, producing a characteristic silver mirror.
In addition to its chemical reactivity, silver nitrate is employed in analytical chemistry for titrations involving halides, cyanides, and thiocyanates. Known as argentometric titrations, these methods rely on the formation of insoluble silver salts to determine the concentration of analytes in solution.
In summary, the mechanism of silver nitrate's action is primarily attributed to the reactivity of silver ions (Ag⁺). These ions participate in a variety of reactions, ranging from antimicrobial activity and photochemical processes to organic and analytical chemistry applications. Silver nitrate's versatility and effectiveness make it a crucial compound in numerous scientific and industrial domains.
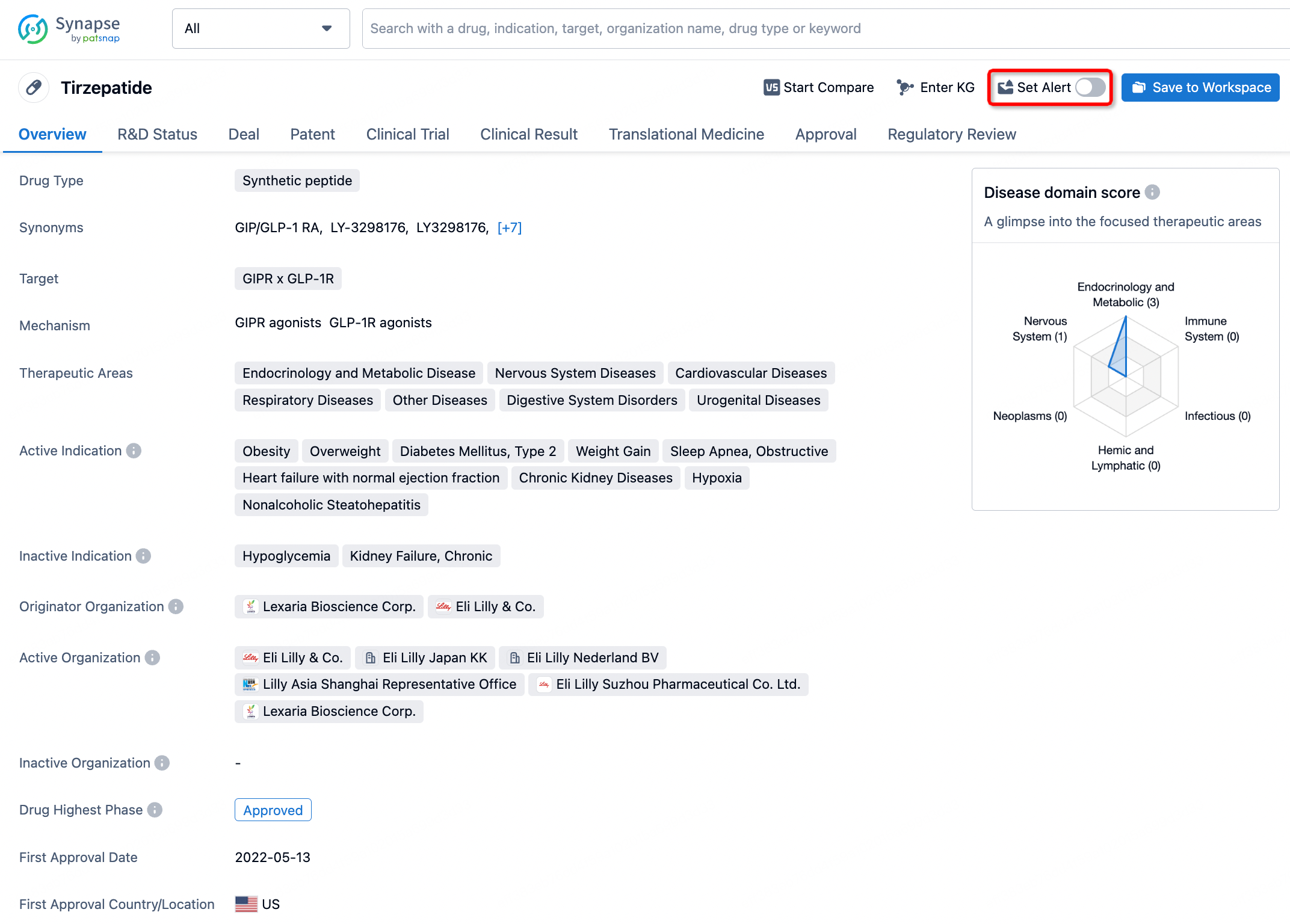
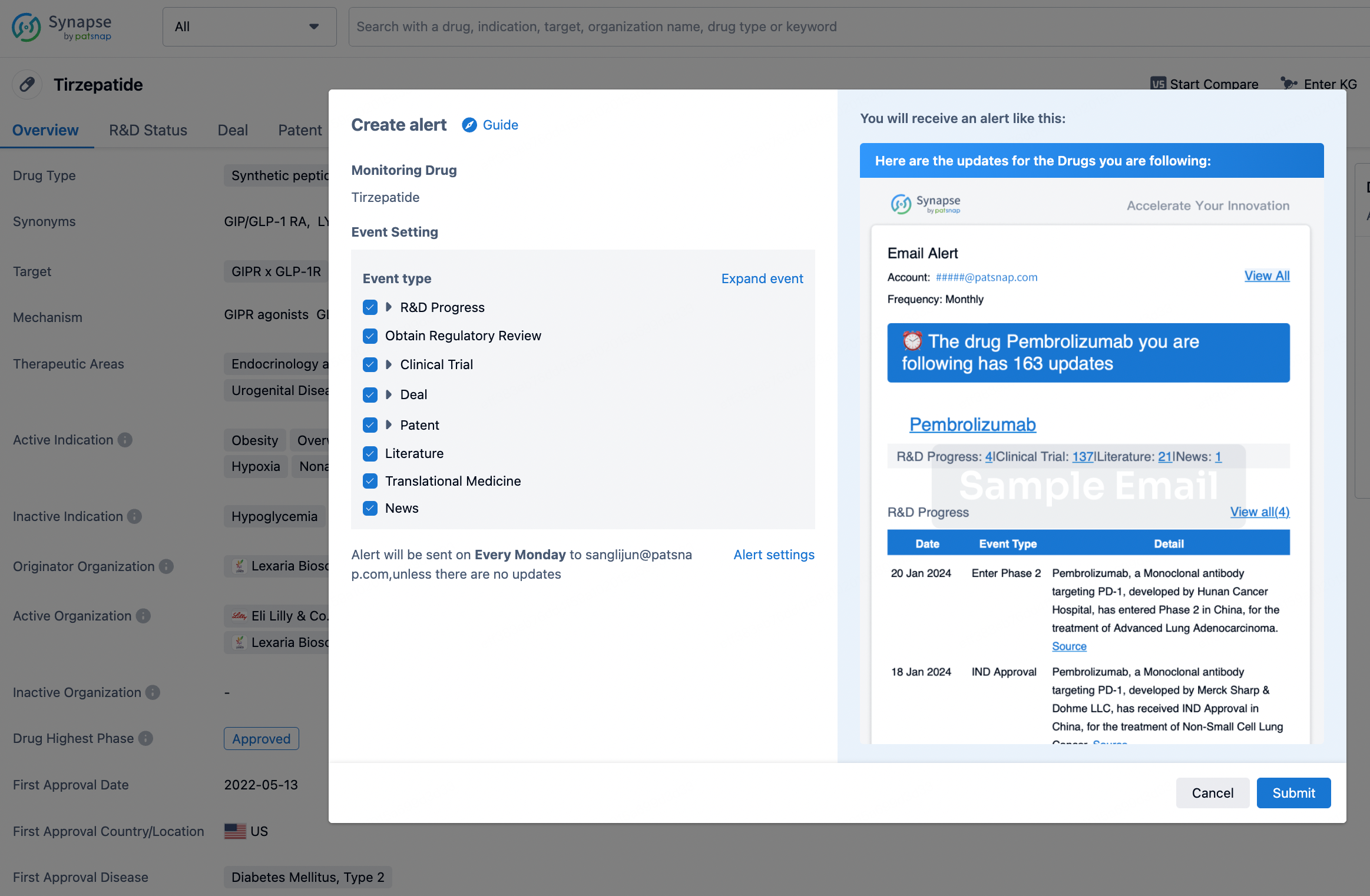
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.