A Comprehensive Review of Pentostatin's R&D Innovations and Drug Target Mechanism
Pentostatin's R&D Progress
Pentostatin is a small molecule drug that primarily targets adenosine deaminase(ADA). It is used in the treatment of various diseases related to the immune system, hemic and lymphatic systems, and neoplasms. The drug has been approved for the treatment of T-cell leukemia, T-cell lymphoma, and hairy cell leukemia.
Pentostatin was developed by Pfizer Inc., a renowned pharmaceutical company. It received its first approval in the United States in October 1991. The drug has been classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions that affect a small number of patients.
As a small molecule drug, Pentostatin is designed to interact with ADA, an enzyme involved in the breakdown of adenosine. By inhibiting ADA, Pentostatin helps to regulate the levels of adenosine in the body, which can have a significant impact on the immune system and the growth of cancer cells.
The therapeutic areas in which Pentostatin is used highlight its potential in treating diseases related to the immune system, such as neoplasms, immune system diseases, and hemic and lymphatic diseases. T-cell leukemia, T-cell lymphoma, and hairy cell leukemia are the active indications for whichPentostatin has been approved.
The fact that Pentostatin has reached the highest phase of development, which is approval, indicates that it has successfully undergone rigorous testing and evaluation to demonstrate its safety and efficacy. This milestone is a significant achievement for Pfizer Inc. and highlights the potential of Pentostatin as a treatment option for the specified indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pentostatin: ADA Inhibitors
ADA inhibitors are a type of medication that inhibits the activity of the enzyme ADA. Adenosine deaminase is an enzyme involved in the breakdown of adenosine, a nucleoside that plays a role in various physiological processes in the body.
From a biomedical perspective, ADA inhibitors are primarily used in the treatment of certain medical conditions, such as chronic lymphocytic leukemia (CLL) and hairy cell leukemia. In these conditions, ADA inhibitors help to reduce the levels of adenosine, which can be elevated and contribute to the progression of the disease.
By inhibiting ADA, these medications increase the levels of adenosine in the body, which can have various effects. Adenosine has anti-inflammatory properties and can also modulate the immune response. Therefore, ADA inhibitors can be beneficial in reducing inflammation or modulating the immune system.
It's important to note that ADA inhibitors should be used under the guidance of a healthcare professional, as they can have potential side effects and interactions with other medications.
Drug Target R&D Trends for Pentostatin
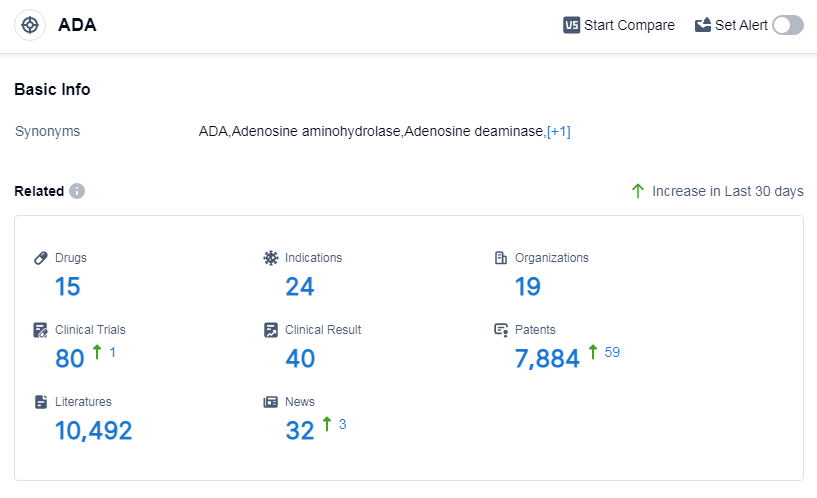
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 15 ADA drugs worldwide, from 19 organizations, covering 24 indications, and conducting 80 clinical trials.
Based on the analysis of the data, the current competitive landscape of target ADA shows that Orchard Therapeutics Plc is leading in terms of R&D progress, with the highest number of drugs in the approved stage. The indication analysis reveals that drugs under the target ADA have been approved for various indications, including adenosine deaminase deficiency, dyspnea, T-cell lymphoma, and severe combined immunodeficiency. The drug types that are progressing most rapidly are small molecule drugs and gene therapy, indicating intense competition in the market. The countries/locations that are developing fastest under the target ADA are the European Union, the United States, France, Japan, Italy, and China. Overall, the target ADA has a promising future with ongoing research and development efforts in multiple companies and countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pentostatin is a small molecule drug developed by Pfizer Inc. that targets ADA. It has been approved for the treatment of T-cell leukemia, T-cell lymphoma, and hairy cell leukemia. The drug's first approval was obtained in the United States in 1991, and it is classified as an orphan drug. Pentostatin shows promise in the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. Its approval signifies its successful development and potential as a therapeutic option in the pharmaceutical industry.