Unleashing the Power of Nitisinone: A Comprehensive Review on R&D Breakthroughs
Nitisinone's R&D Progress
Nitisinone is a small molecule drug that targets 4HPPD, which stands for 4-hydroxyphenylpyruvate dioxygenase. It is primarily used in the treatment of various conditions related to the nervous system, congenital disorders, and endocrinology and metabolic diseases. The active indications for Nitisinone include alkaptonuria, tyrosinemias, and tyrosinemia type I.
The drug was developed by Swedish Orphan Biovitrum AB, a pharmaceutical company specializing in rare diseases and orphan drugs. Nitisinone has received approval for use in multiple countries, including the United States, where it was first approved in January 2002. The drug has also obtained approval in China.
Nitisinone is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of individuals. This designation provides certain incentives and benefits to the drug manufacturer, such as extended market exclusivity and financial support for research and development.
The highest phase of development for Nitisinone is approved, indicating that it has successfully completed clinical trials and demonstrated its safety and efficacy. As a small molecule drug, Nitisinone is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nitisinone: 4HPPD inhibitors
4HPPD inhibitors are a type of chemical compounds that inhibit the activity of the enzyme 4-hydroxyphenylpyruvate dioxygenase (4HPPD). From a general understanding, these inhibitors work by binding to the active site of the enzyme and preventing its normal function.
From a biomedical perspective, 4HPPD inhibitors have been studied and used in the field of herbicides. They are commonly used to control weeds in crops by inhibiting the enzyme 4HPPD, which is essential for the synthesis of certain pigments in plants. By inhibiting this enzyme, the inhibitors disrupt the normal metabolic processes in plants, leading to their growth inhibition or death.
It's important to note that the use of 4HPPD inhibitors as herbicides is specific to the field of agriculture and plant science, and their application in biomedicine or clinical settings is limited.
Drug Target R&D Trends for Nitisinone
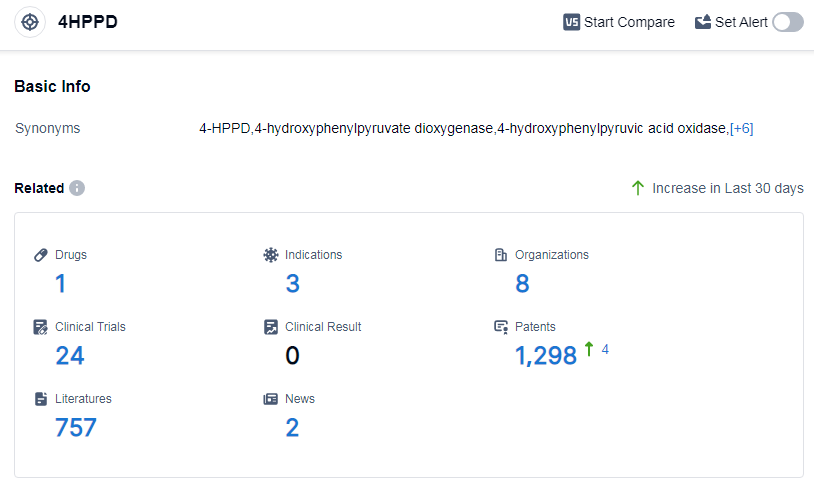
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 1 4HPPD drugs worldwide, from 8 organizations, covering 3 indications, and conducting 24 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for target 4HPPD involves several companies, including MendeliKABS Inc., Cycle Pharmaceuticals Ltd., Chinese Medicines(Guangzhou)Ltd, Astellas Pharma, Inc., Laboratoires K.A.B.S., Inc., Supi Pharmaceutical (Guangzhou) Co., Ltd., and Swedish Orphan Biovitrum AB. These companies have drugs in various stages of development, with MendeliKABS Inc. and Cycle Pharmaceuticals Ltd. leading the way.
The indications for drugs targeting 4HPPD primarily focus on metabolic disorders such as alkaptonuria and tyrosinemia. Small molecule drugs are progressing rapidly in the development of drugs targeting 4HPPD.
The countries/locations that are developing fastest under the current target include the United States, European Union, Japan, and China. China's progress in this area indicates its active involvement in the development of drugs targeting 4HPPD.
Overall, the current competitive landscape for target 4HPPD is dynamic, with multiple companies and countries actively contributing to the research and development of drugs. The future development of target 4HPPD holds promise, particularly in the treatment of metabolic disorders, with a focus on small molecule drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nitisinone is a small molecule drug developed by Swedish Orphan Biovitrum AB. It targets 4HPPD and is used in the treatment of various nervous system diseases, congenital disorders, and endocrinology and metabolic diseases. The drug has been approved in multiple countries, including the United States and China, and it received its first approval in 2002. Nitisinone is classified as an orphan drug, indicating its focus on treating rare diseases.