A new approach to the development of targeted drugs: PROTACs
Proteolysis Targeting Chimeras (PROTACs) technology is a new protein degradation strategy that has emerged in recent years. It utilizes bifunctional small molecules to induce the ubiquitination and degradation of target proteins through the ubiquitin-proteasome system. PROTACs can not only serve as potential clinical treatments for diseases such as cancer, immune disorders, viral infections, and neurodegenerative diseases, but can also provide unique tools for biological research in a catalytic, reversible, and rapid manner.
Advantages of PROTACs
1. Wider targeting range:
Traditional small molecules and antibodies are all act on diseases through an "occupancy-driven" model by inhibiting the functions of target proteins. This functioning model requires either high concentrations of inhibitors or monoclonal antibodies to occupy the target's active sites and thus block the transduction of downstream signalling pathways. In contrast, PROTACs are "event-driven", and instead of affecting the protein's function, they mediate the degradation of disease-causing target proteins. As long as PROTACs induce the formation of a ternary complex and ubiquitinate the target protein, it can theoretically be used cyclically and repetitively, thus even small catalytic amounts can have an effect. Moreover, PROTACs can significantly broaden the range of targets, as they can induce protein degradation through binding alone, even when the protein has no active sites (e.g. scaffold proteins).
Targets suitable for PROTACs typically have four characteristics:
i) "Undruggable";
ii) Abnormal protein expression (including overexpression, mutation, abnormal aggregation, misfolding, etc.);
iii) Scaffold proteins;
iv) Resistant to existing therapies and drugs.
2. Higher selectivity and safety:
In comparison to traditional small molecule inhibitors, PROTACs can achieve levels of selectivity that are difficult for small molecules at some targets. As is widely known, small molecule inhibitors often target multiple sites, for example, kinase inhibitors often use 2,4-diaminopyrimidine as a scaffold, which is used in the inhibitors of EGFR, ALK, CDK, Jak, etc. Typically, these drug molecules have poor target selectivity, and often exhibit strong inhibitory activity on various other kinases. Off-target effects often become the main source of toxicity and side effects in drug development, affecting the success rate of new drug development.
3. Overcoming resistance:
In clinical practice, it is inevitable that acquired resistance will occur with the use of small molecule inhibitors or antagonists. For instance, instances of resistance like EGFR-T790M and C797S. Although this issue of resistance can be addressed by developing new generations of inhibitors, like third and fourth generation EGFR inhibitors, new resistances will come up with the use of new generation drugs. PROTAC technology has already shown some advantages in overcoming resistance. Several research groups around the world are trying to use PROTACs for EGFR, hoping to overcome drug-resistant mutations or explore breakthrough protein degradation therapies for inhibitors. Studies have shown that the first, second, third, and fourth generation inhibitors, as ligands binding target protein EGFR, have all been applied in PROTAC design.
In terms of antiviral actions, PROTACs also have a role to play. The feasibility of antiviral PROTACs molecules was initially established in research into the degradation of Hepatitis C virus (HCV) NS3/4A protease.
Over the past two decades, PROTACs technology has gradually developed into one of the hottest techniques in modern drug research, attracting extensive attention from research institutions, pharmaceutical companies, and investors. The most advanced PROTACs drug is still in Phase II clinical trials and the pioneers will have to deal with a host of issues in pharmacology, toxicology, biochemical characteristics, and even commercialization. Compared to popular fields (cell gene therapy, large molecule drugs), PROTACs still has a great deal of room for development.
PROTACs Competitive Landscape
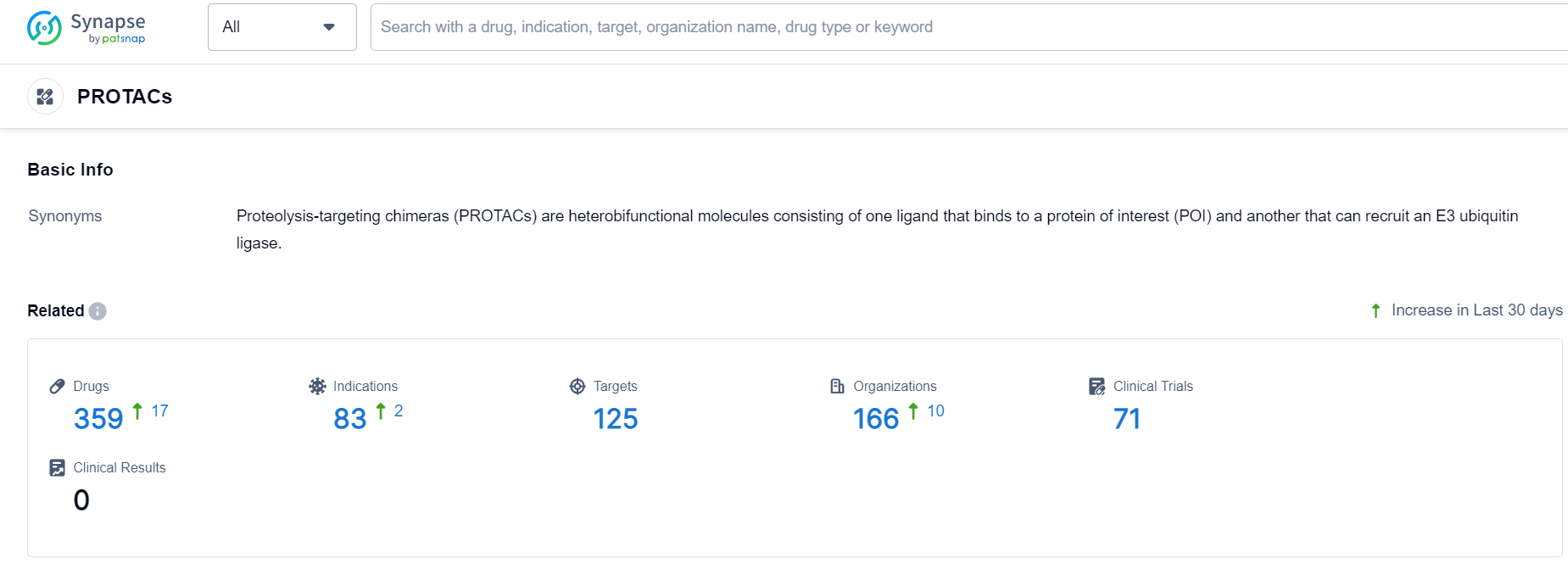
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 359 PROTACs drugs worldwide, from 166 organizations, covering 125 targets, 83 indications, and conducting 71 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
Phase III Clinical Trial of saRNA Medicinal: Vepdegestrant
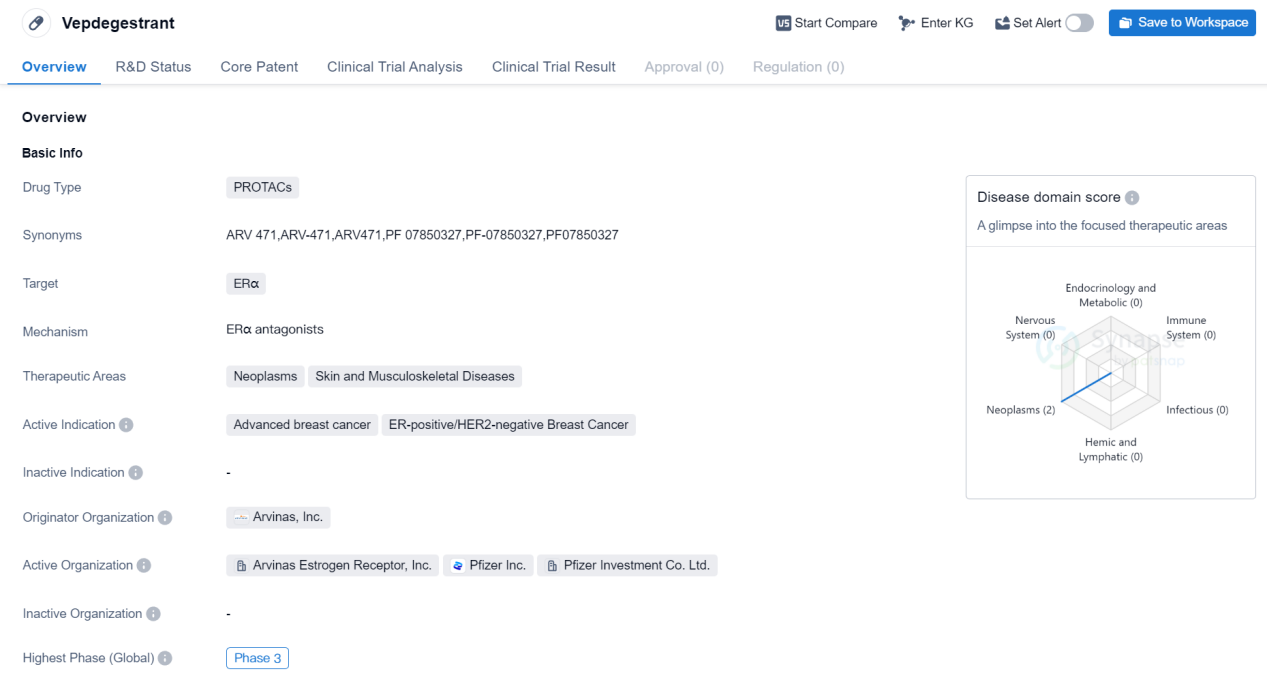
Vepdegestrant is a drug classified as PROTACs, which stands for Proteolysis Targeting Chimeras. This type of drug works by utilizing small molecules to target specific proteins for degradation within cells. In the case of Vepdegestrant, its target is ERα, which is a protein involved in estrogen receptor signaling.
The therapeutic areas for Vepdegestrant include neoplasms, which refers to abnormal growth of cells that can lead to cancer, as well as skin and musculoskeletal diseases. This suggests that the drug may have potential applications in treating various types of cancers, as well as conditions affecting the skin and musculoskeletal system.
The active indication for Vepdegestrant is advanced breast cancer, specifically ER-positive/HER2-negative breast cancer. This indicates that the drug is intended for use in patients with breast cancer that has spread to other parts of the body and is characterized by the presence of estrogen receptors but the absence of HER2 receptors.
Vepdegestrant was developed by Arvinas, Inc., an originator organization in the pharmaceutical industry. The drug has reached the phase 3 of clinical development. Phase 3 trials are large-scale studies conducted to evaluate the safety and efficacy of a drug in a larger patient population before it can be submitted for regulatory approval.
In summary, Vepdegestrant is a PROTACs drug that targets ERα and is being developed by Arvinas, Inc. It has shown potential in the treatment of advanced breast cancer, specifically ER-positive/HER2-negative breast cancer. The drug is currently in Phase 3 clinical trials, indicating that it has progressed through earlier stages of development and is being evaluated in larger patient populations.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Phase II Clinical Trial of saRNA Medicinal: GT20029
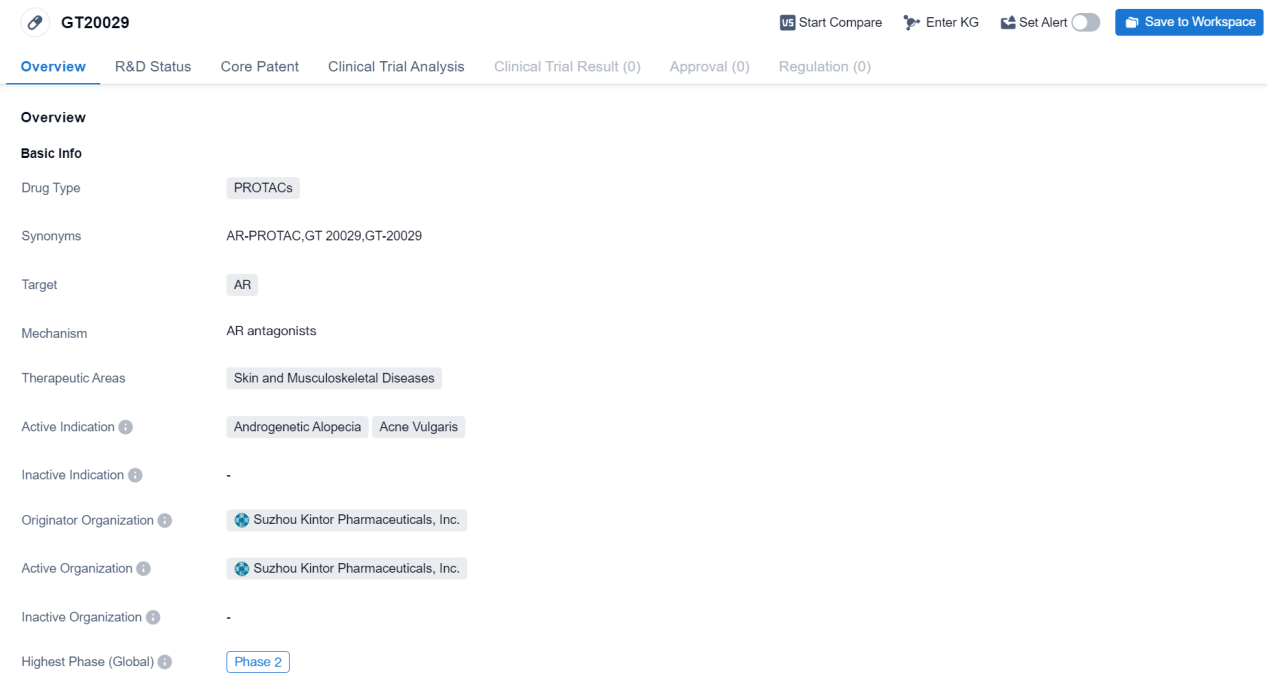
The drug GT20029 is a type of PROTACs, which stands for Proteolysis Targeting Chimeras. PROTACs are a new class of drugs that work by harnessing the body's own protein degradation system to selectively remove disease-causing proteins. In the case of GT20029, it targets the androgen receptor (AR), which is involved in the development and progression of various skin and musculoskeletal diseases.
The therapeutic areas for GT20029 are focused on skin and musculoskeletal diseases. Specifically, it is being developed for the treatment of androgenetic alopecia, which is a common form of hair loss that affects both men and women, and acne vulgaris, a chronic inflammatory skin condition characterized by pimples, blackheads, and whiteheads.
The drug GT20029 is being developed by Suzhou Kintor Pharmaceuticals, Inc., an originator organization based in China. It has reached the phase 2 of clinical development. Phase 2 trials involve a larger number of patients and aim to further evaluate the safety and efficacy of the drug in the target population.
Based on the available information, GT20029 shows promise in the treatment of androgenetic alopecia and acne vulgaris. The use of PROTACs as a novel therapeutic approach offers the potential for more targeted and effective treatments for these conditions. However, further clinical trials and research are needed to fully assess the safety and efficacy of GT20029.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, GT20029 is a PROTACs drug being developed by Suzhou Kintor Pharmaceuticals, Inc. It targets the androgen receptor and is intended for the treatment of androgenetic alopecia and acne vulgaris. The drug has reached Phase 2 of clinical development. Further research and trials are required to determine its full potential in treating these skin and musculoskeletal diseases.