AACR 2024: Crescendo Biologics Unveils CB307 Data and Debuts Dual-Action Immune Attractor CB699
At the 2024 Annual Meeting of the American Association for Cancer Research, Crescendo Biologics Ltd, a biopharmaceutical entity in the clinical phase focusing on the creation of unique therapeutics that boost immune cell functions for cancer treatment, showcased preliminary findings from its two major initiatives.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The experimental treatment, CB307, stands out as a pioneering therapeutic option in the realm of CD137 x PSMA bispecifics tailored for patients with advanced prostate cancer. Additionally, Crescendo's innovative product, CB699, serves as the premier bifunctional immune cell engager targeting mesothelin, operating via dual engagement of CD40 and CD137.
Sourced from the novel Humabody® VH technology by Crescendo, these pioneering treatments boast significantly enhanced penetration into tumors in comparison to conventional antibody therapies.
At the helm of Crescendo Biologics, Theodora Harold, the company's CEO, remarked on their strategic focus on tumor-centric modulation of immune responses to combat cancer. She emphasized the promising nature of their flagship immuno-oncology treatments, underscoring their effectiveness, durability, and overall patient compatibility, as demonstrated by the preliminary clinical data.
Harold highlighted the steady progression of CB307 within an active Phase 1b study targeting PSMA+ metastatic castration-resistant prostate cancer, underpinned by promising pharmacological findings. She expressed enthusiasm regarding the progress of CB699, crediting the early experimental results discussed at a recent conference, which firmly support its advancement into clinical trials. The management team eagerly anticipates sharing additional updates as the research on this candidate further unfolds.
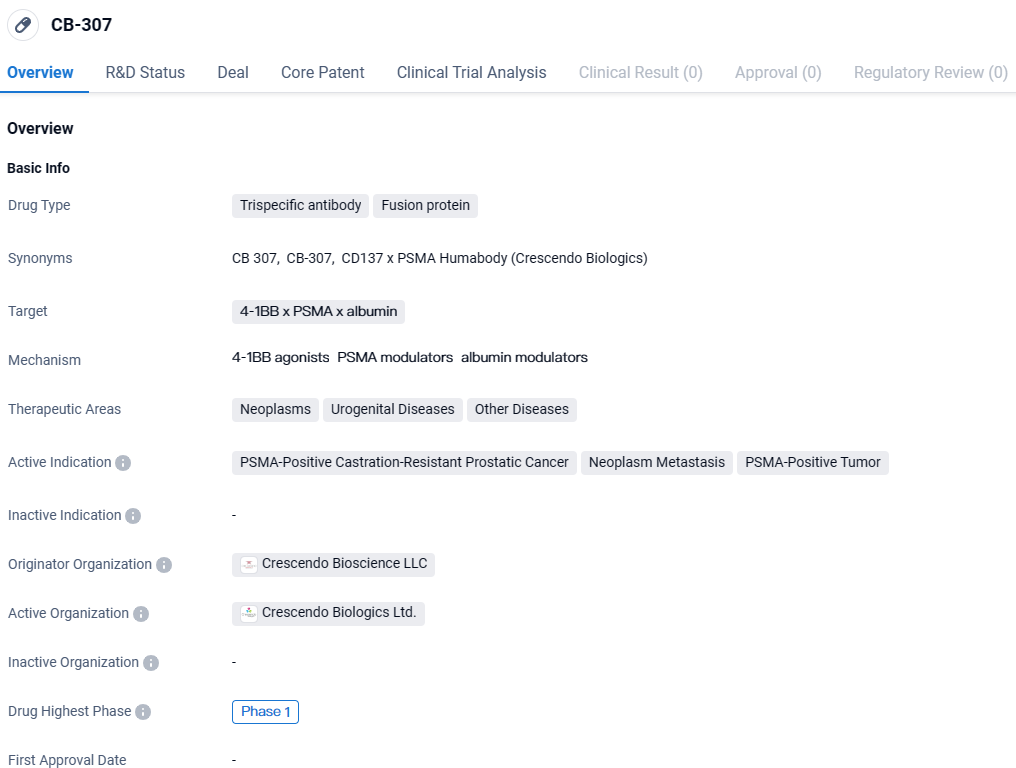
CB307 is at the forefront of Crescendo’s developmental pipeline, characterized as a novel and extended half-life CD137 x PSMA bispecific Humabody®. This therapeutic is engineered to enhance the efficacy and safety of anti-cancer effects.
The medication is formulated to selectively activate cancer-specific T cells within the tumor microenvironment through the CD137 co-stimulatory pathway. Its innovative design delivers focused and robust anti-tumor activity, circumventing the risk of systemic adverse effects. This offers a promising treatment route for a spectrum of PSMA+ malignancies, thereby fulfilling a significant gap in healthcare.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
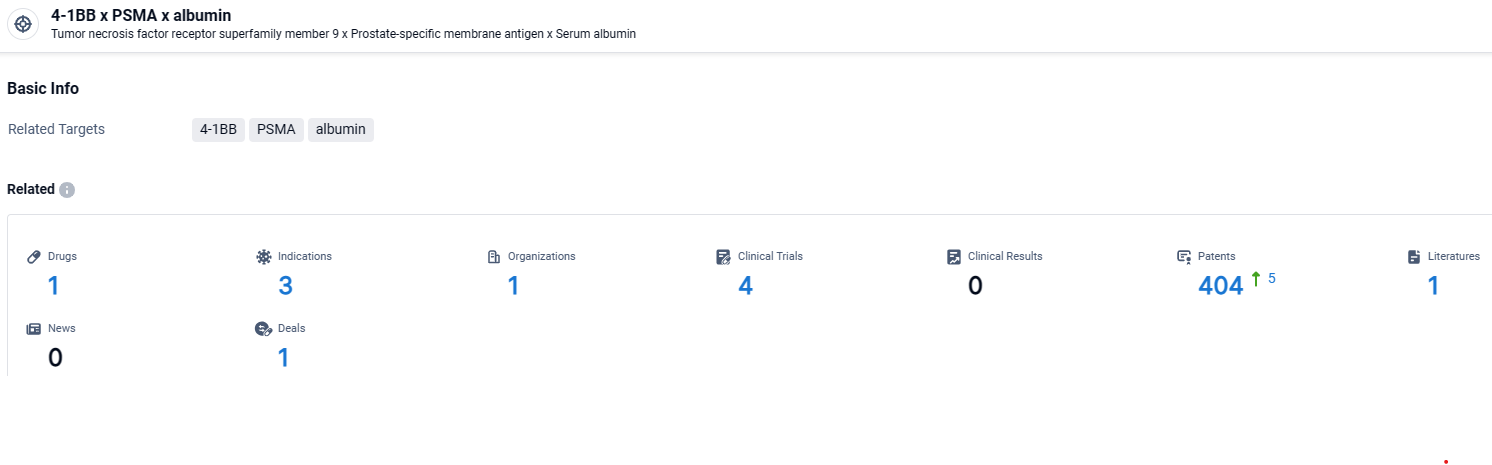
According to the data provided by the Synapse Database, As of April 15, 2024, there are 1 investigational drugs for the 4-1BB and PSMA and albumin target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 4, and as many as 404 patents.
CB-307 targets 4-1BB, PSMA, and albumin and is being investigated for its potential in treating neoplasms, urogenital diseases, and other diseases. The drug is currently in Phase 1 of clinical development and is primarily indicated for PSMA-positive castration-resistant prostatic cancer, neoplasm metastasis, and PSMA-positive tumor.