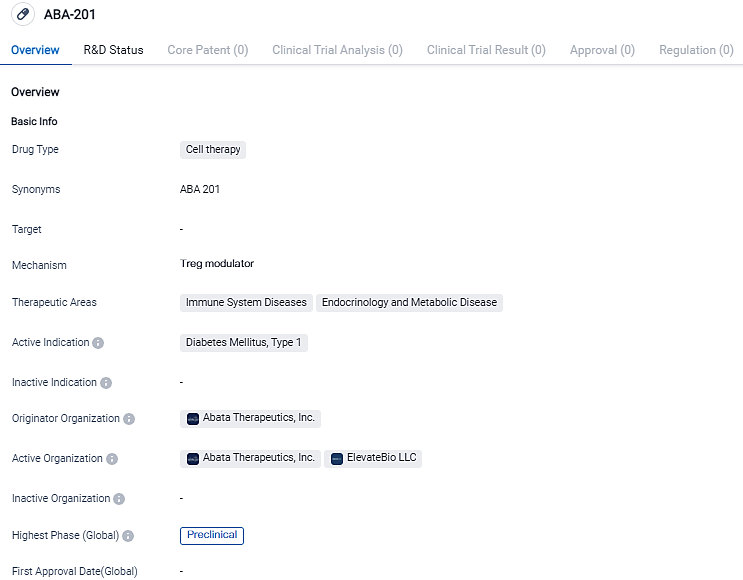
Abata Therapeutics Unveils ABA-201, a New Development Candidate for Type 1 Diabetes Management
Abata Therapeutics, devoted to transforming the science of regulatory T cells into innovative treatments for individuals suffering from acute autoimmune and inflammatory conditions, unveiled its second development candidate today - ABA-201. It holds the promise of being a potent modifying Treg cell therapy for type 1 diabetes patients who still possess beta (β) cell functionality.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Samantha Singer, M.S., M.B.A., President and Chief Executive Officer of Abata, applauds the impressive progress their team has made in developing the Treg cell therapy for treatment. Within a mere couple of years, they've brought forward two unique therapeutic options, aimed at addressing two severe autoimmune disorders in desperate need of treatment.
Expressing excitement about the likely commencement of ABA-201’s clinical trials for T1D in 2025, she emphasized how this achievement rests on the foundational work done on their premier program, ABA-101. This drug is slated for treating escalating multiple sclerosis and is scheduled to enter clinical trials the upcoming year.
She also expressed satisfaction at the continuation of their joint venture with ElevateBio, emphasizing the role it has played in their primary program and positing how this symbiotic relationship would fast-track the progression of their ABA-201 program.
The rationale behind proposing ABA-201 as a Treg remedy for T1D is firmly grounded in scientific research. Evidence collected from preclinical models of insulitis and clinical trials testing immunomodulators or polyclonal Tregs in patients with at-risk T1D underlines the potential potency of autoantigen-specific Tregs in treating T1D.
Further non-clinical research consolidates the contribution of Tregs to mitigating β-cell damage, thereby reinforcing the potential benefits of this treatment methodology.
According to Leonard Dragone, M.D., Ph.D., Chief Medical Officer of Abata, ABA-201, a Treg cell therapy, targets the pancreas and draining lymph nodes in T1D patients, with the goal to conserve remaining β-cell mass and clamp-down continuing β-cell decay.
He emphasizes the lack of a durable treatment to cease the autoimmunity propelling T1D entirely and expressed his excitement to test these candidates for autoimmune afflictions he's worked his career to treat.
Steven St. Peter, M.D., Managing Director of JDRF T1D Fund, a subset of the primary T1D research foundation, JDRF International, talked about how the treatment of T1D has been a longstanding objective for the Fund since its inception.
As he appreciates the potential of Treg-based therapeutics in treating T1D and thereby improving patient outcomes, he added his anticipation of supporting Abata and ABA-201 in the foreseeable future.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
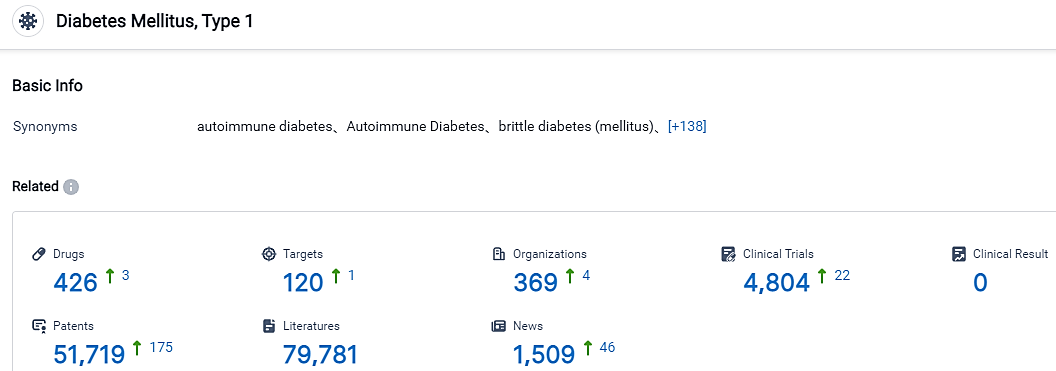
According to the data provided by the Synapse Database, As of September 4, 2023, there are 426 investigational drugs for the type 1 diabetes, including 120 targets, 369 R&D institutions involved, with related clinical trials reaching 4804, and as many as 51719 patents.
ABA-201, an autologous Treg therapy developed by Abata, is designed to treat Type 1 Diabetes. Its aim is to target T1D individuals with retained beta cell functionality and a specific HLA genetic haplotype. By minimally modifying the patient's own Tregs to express a TCR that specifically recognizes immunogenic protein fragments in the pancreas and draining lymph nodes, ABA-201 is generated.
This tactic potentially offers robust safety aspects and a localized anti-inflammatory response at the disease site. When tested in vitro, ABA-201 has proven to reveal antigen-specific, dose-dependent Treg functionality, alongside anti-inflammatory cytokine production and inhibition of inflammatory factor production. On an annual basis, new T1D cases in the United States are projected to reach approximately 64,000.