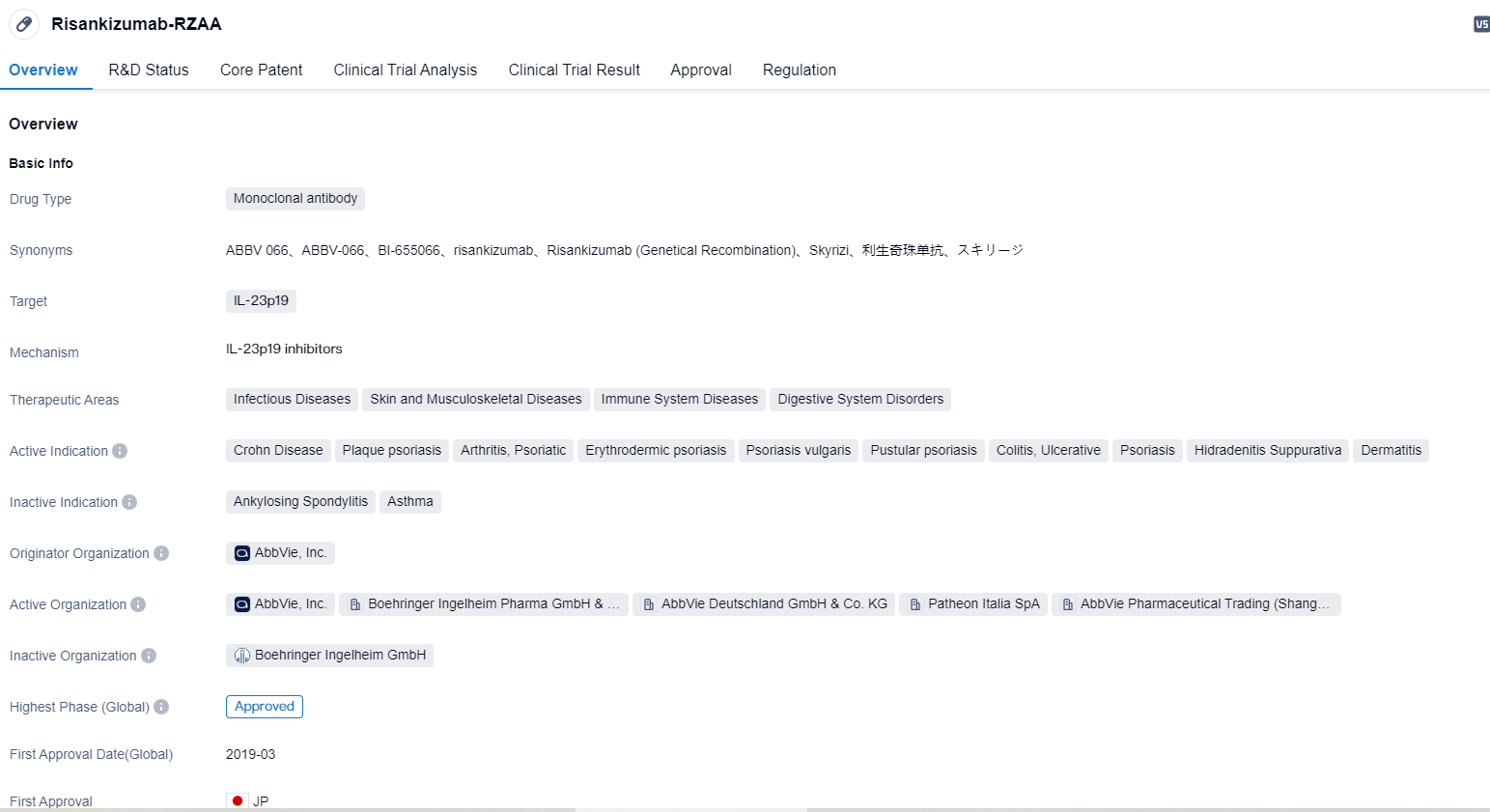
AbbVie has lodged filings with the FDA and EMA for approval of Risankizumab (SKYRIZI®) to treat Ulcerative Colitis
The backing for these application materials comes from two Phase 3 clinical studies which showcased that risankizumab fulfilled the main objective of achieving clinical remission (as per Adapted Mayo Score) in addition to crucial secondary objectives for both induction and maintenance treatment. Safety outcomes were largely in line with risankizumab's known safety characteristics, with no newly noticed safety concerns. This IL-23 inhibitor, risankizumab, is being tested as a potential remedy for adults suffering from moderately to severely active ulcerative colitis, and it's already approved by the FDA and EMA for Crohn's disease, psoriatic arthritis, and psoriasis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
AbbVie (NYSE: ABBV) unveiled that they have sent applications for a fresh indication to the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for risankizumab, consisting of doses of 1200 mg intravenous [IV] [induction dose] and subcutaneous doses of 180 mg and 360 mg [SC] [maintenance dose], to be used for treating adult patients dealing with moderately to severely active ulcerative colitis.
"While progress has been made in treatments for ulcerative colitis, the quest for more therapies to alleviate its debilitating effects continues," stated Roopal Thakkar, M.D., the Senior Vice President of development, regulatory affairs, and Chief Medical Officer at AbbVie. “The applications we've made reflect our persistent dedication to aiding people suffering from IBD, and we're excited about potentially providing a new therapy choice for managing ulcerative colitis.”
Applications to the FDA and EMA are bolstered by information from two phase 3 clinical trials: an initiation trial, INSPIRE, and a continuation trial, COMMAND.1,2 Patients receiving risankizumab 1200 mg IV at week 12 in the initiation trial and 180 mg or 360 mg SC at week 52 in the continuation trial had a significantly higher rate of achieving the primary goal of clinical remission compared to patients given a placebo. Furthermore, risankizumab-treated patients in both trials showed higher attainment of the crucial secondary goals of endoscopic improvement (endoscopic subscore ≤1 without sign of fragility) and histologic endoscopic mucosal enhancement (HEMI, defined as endoscopic subscore ≤1 without sign of fragility and a Geboes score ≤3.1) in comparison to placebo.
The safety outcomes in INSPIRE and COMMAND were generally in line with the safety profile of risankizumab seen in previous trials across different conditions, with no new safety risks spotted.
Risankizumab (SKYRIZI) is a joint endeavor between Boehringer Ingelheim and AbbVie, with AbbVie spearheading development and worldwide commercialization.
About Ulcerative Colitis
Ulcerative colitis is a chronic, autoimmune inflammatory bowel disease (IBD) of the large intestine, instigating continuous mucosal inflammation that extends to varying degrees from the rectum to the more proximal sections of the colon.3,4 The characteristic signs of ulcerative colitis include rectal bleeding, abdominal discomfort, bloody diarrhea, a pressure feeling (tenesmus), urgency, and fecal incontinence.3,4 The progression of ulcerative colitis differs among patients and can span from dormant disease to chronic resistant disease, potentially leading to surgery or life-threatening complexities in some circumstances.5,6 The unpredictability of the disease course and the severity of the symptoms can impose a substantial burden and often result in disability among individuals living with the disease.
Regarding the INSPIRE Induction Study1
INSPIRE is a globally distributed, stratified, concealed testing, placebo-based Phase 3 trial examining the effectiveness and safety of risankizumab 1200 mg IV, given every four weeks as initial treatment to participants with medium to severe active ulcerative colitis.
The foremost result marker is clinical remission (according to changed Mayo Score, recognized as SFS ≤1 and not exceeding the baseline, RBS of 0 and endoscopic subscore ≤1 without friability) by the 12th week. Principal auxiliary tract markers involve clinical reaction (reduction from the initial in the Modified Mayo score by ≥2 points and ≥30% from the benchmark, along with decrease in RBS by ≥1 or a complete RBS ≤1), endoscopic progress (endoscopic subscore ≤1 minus friability), and HEMI (endoscopic subscore either 0 or 1 devoid of friability and Geboes score ≤3.1) by the 12th week.
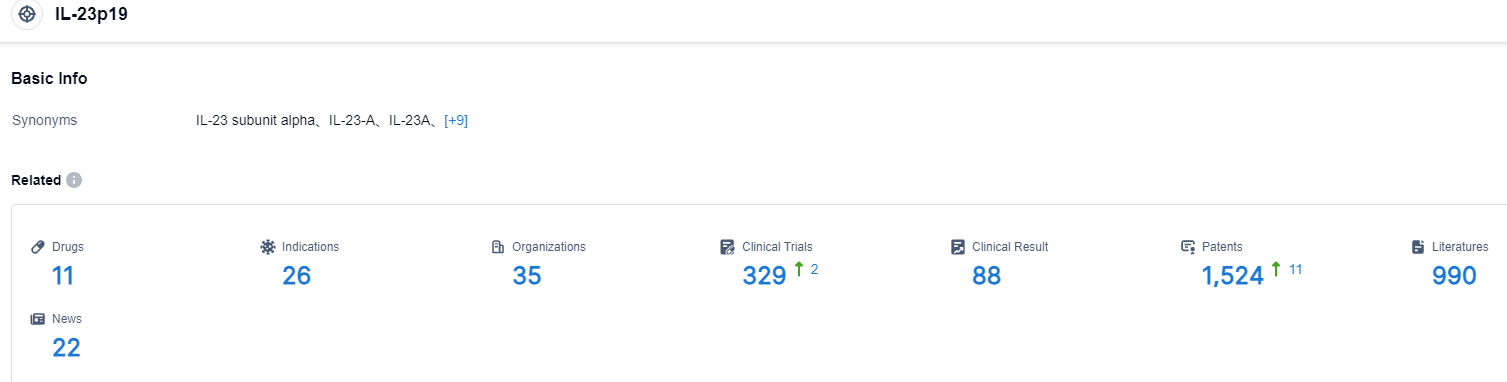
Click on the image below for direct access to the latest R&D progress on IL-23 target drugs, indications, research institutions, clinical trials, and more. as of August 30, 2023, there were 11 drugs under research for the IL-23 target, covering 28 indications, with 35 research institutions involved, and it pertained to 88 related clinical trials and as many as 1524 patents. Risankizumab (AbbVie/Boehringer Ingelheim) is a humanized anti-interleukin (IL)-23 subunit p19 monoclonal antibody that binds and neutralizes the p19 subunit of IL-23. It is being developed in psoriasis and Crohn’s disease.