The primary objective of BlueRock's Phase I trial involving bemdaneprocel for individuals suffering from Parkinson's disease has been successfully achieved
The investigational cell treatment, bemdaneprocel (BRT-DA01), showed good tolerance and no major safety concerns throughout a year in 12 subjects belonging to both low and high dose groups.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
After 12 months, the experimental clinical endpoints generally showed improvement, with those subject in the high dose groups illustrating substantial enhancement.
An annual evaluation of all subjects validated the practicality of transplantation, along with the survival and engraftment of the cells.
Omega preparations are being made for a Phase II trial, which is anticipated to begin recruiting new members in H1 (the first six months) of 2024.
Bayer AG and BlueRock Therapeutics LP, a cell therapy company in clinical phase and a subsidiary of Bayer AG operating independently, disclosed specifics of the promising outcomes from the Phase I clinical trials of bemdaneprocel (BRT-DA01), a stem cell derived experimental treatment for Parkinson's disease. The presented data was from the International Congress of Parkinson's Disease and Movement Disorders® held in Copenhagen, Denmark.
The research accomplished its primary aim of displaying safety and tolerability in each of the 12 participants across the low and high dose groups in the study, with no serious adverse events (SAEs) associated with bemdaneprocel being reported over the course of a year. Two SAEs were reported, however, neither were linked to bemdaneprocel, with one seizure being ascribed to the surgical procedure and one COVID case. Both were resolved without lasting effects. Furthermore, 18F-DOPA PET imaging scans provided proof of cell survival and engraftment in both the low and high dose groups. 18F-DOPA PET imaging is a neuroimaging procedure used to examine and evaluate dopaminergic activity in Parkinson's disease.
Secondary investigational clinical endpoints exhibited improvement in both groups, with members of the high dosage group indicating a higher level of improvement as assessed by the MDS-Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS Part III) and the Hauser Diary, which are measures used to evaluate the severity of Parkinson's disease motor symptoms.
"The findings from our Phase I open label investigation carry a great deal of optimism," stated Claire Henchcliffe, MD, head of the Department of Neurology at the UCI School of Medicine, University of California, Irvine, and one of the Principal Investigators. “Granting that this is a fairly small open label research, accomplishing the research's main targeted outcome concerning safety and tolerability, along with the initial enhancements seen in clinical results, is a significant progression. The aspiration now is that these tendencies endure and bring forth considerable benefits for individuals with Parkinson’s in controlled clinical experiments.”
Utilizing the Hauser Diary, which classifies patients as being in the "ON" phase when their symptoms are effectively controlled and in the "OFF" phase when there is a symptom deterioration, individuals in the high dosage group displayed an enhancement of 2.16 hours in the duration spent in the "ON" phase devoid of troubling dyskinesia compared to the baseline after one year. The time spent in the "OFF" phase demonstrated a corresponding decrease of 1.91 hours after one year. Participants in the low dosage group showed a development of 0.72 hours in the "ON" phase free of troubling dyskinesia compared to baseline and a corresponding decrease of 0.75 hours in "OFF" phase.
In the high dosage group, year-long measurement of the impacts of bemdaneprocel using MDS-UPDRS Part III assessed during the "OFF"-medication state, exhibited a reduction of 13.0 points compared with baseline. The low dosage group displayed a reduction of 7.6 points.
"The urgency for novel therapeutic interventions to assist patients grappling with Parkinson’s disease is evident," expressed Ahmed Enayetallah, Senior Vice President and Head of Development at BlueRock Therapeutics. "We eagerly anticipate sharing the outcomes of this Phase I study and advancing bemdaneprocel to the subsequent stage of clinical examination."
Anticipating these results, preparations are being made for a Phase II study which is projected to start accepting patients in H1 2024.
"The existing care standards for the millions dealing with Parkinson’s has offered scarcely any improvement over the recent decades, and the current unmet health necessity will only escalate with the expanding aging demographic," stated Christian Rommel, Member of the Executive Committee of Bayer’s Pharmaceuticals Division and Head of Research and Development. "This Phase I clinical study’s positive results are indeed a significant advance, bringing us a step closer to providing new treatment possibilities for patients."
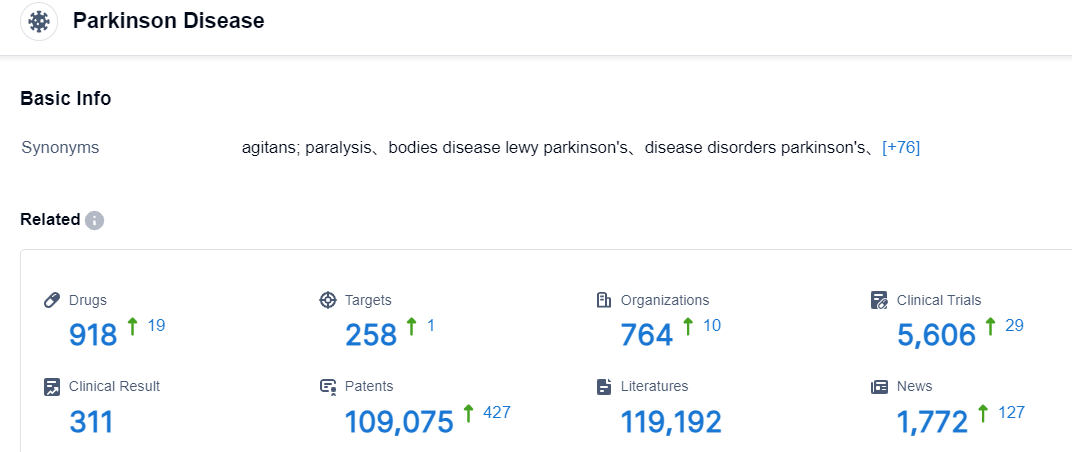
Click on the image below for direct access to the latest R&D progress on bemdaneprocel, indications, research institutions, clinical trials, and more, as of August 30, 2023, Parkinson Disease has a total of 918 drugs under research, involves 258 different targets, 764 institutions conducting research, related to 5606 clinical trials, and has as many as 109075 patents.Despite levodopa’s status as the drug of choice in PD, long-term use frequently results in diminished efficacy and the development of motor fluctuations and dyskinesia.Drugs that are both effective in advanced PD and have improved side-effect profiles are therefore one of the greatest commercial opportunities for drug developers. Other than Nuplazid, which is the first US-approved treatment for PDP, there is little competition regarding advanced PD symptoms, both motor and non-motor.Competition in the rescue therapy space has begun to heat up with the approval of Inbrija and many adjunctive therapies, and this segment will continue to grow as developers look to target the rapid treatment of “off” periods. Continuous infusions have also emerged to tackle this issue, mimicking the continuous release of dopamine in non-pathological conditions. These therapies have been the most efficacious in reducing “off” time and increasing quality “on” time. Developers have also incorporated complex devices/formulations to extend market exclusivity and combat generic imitations.