AC Immune Advances in Phase 2b Trial for Early Alzheimer’s, Receives Further Payment
AC Immune SA (NASDAQ: ACIU), a biopharmaceutical company in the clinical stage focusing on precision therapeutics for neurodegenerative disorders, announced that it will obtain the second milestone payment related to ReTain, totaling CHF 24.6 million, according to its contract with Janssen Pharmaceuticals, Inc. (Janssen), a subsidiary of Johnson & Johnson.
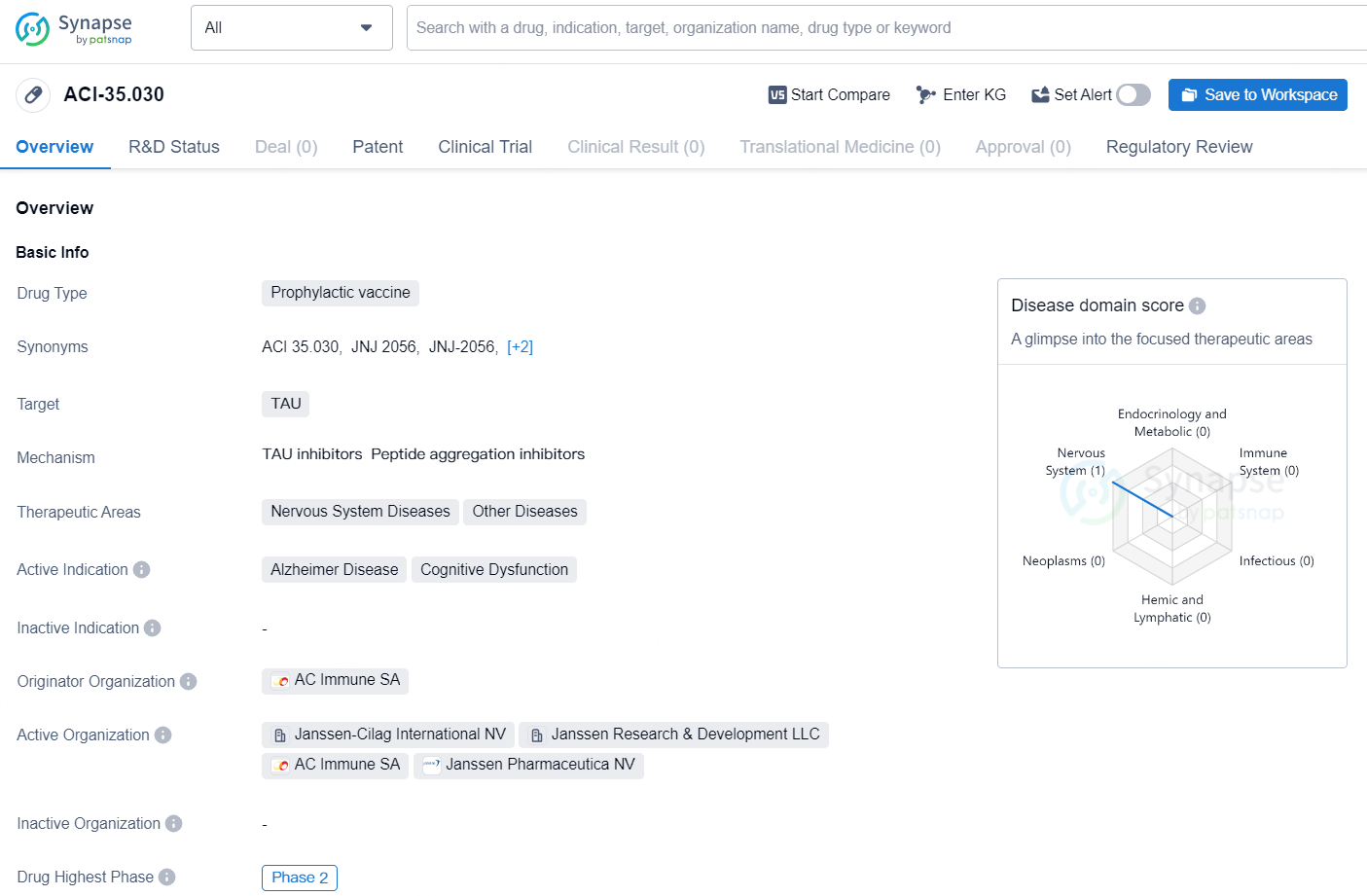
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The milestone payment was initiated due to the swift prescreening rate in the potentially registrational Phase 2b ReTain trial evaluating the active-immunotherapy candidate ACI-35.030 (renamed “JNJ-2056”) for preclinical (pre-symptomatic) Alzheimer's disease (AD). Including the milestone payment from last December, this totals CHF 40 million received for ACI-35.030 related to this trial.
Dr. Andrea Pfeifer, CEO of AC Immune SA, stated: “This early milestone indicates that the medical community and the public share our belief that second-generation therapeutics for Alzheimer’s disease, such as our active immunotherapy targeting pathological phosphorylated-Tau protein (pTau), could offer significant benefits for those diagnosed early, before symptoms emerge. Early diagnosis and treatment are crucial to battle neurodegeneration.”
“This payment also reinforces the excellence and productivity of AC Immune’s technology platforms and drug development capabilities. To date, we have collected a total of around CHF 425 million in milestone and upfront payments from all of our collaborations, with over CHF 4.3 billion in potential milestone payments still possible, plus royalties on future sales. Crucially, in these tough financial markets, this milestone payment bolsters our solid financial standing, giving us three years’ worth of operational funds, during which we aim to reach several potentially transformative milestones.”
JNJ-2056 was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in July, highlighting its unique attributes and potential value for patients. It marks the second active immunotherapy from AC Immune to receive this regulatory milestone, following ACI-24.060, which targets Abeta. Additionally, AC Immune’s PI-2620 Tau-PET diagnostic, now in Phase 3 development, also obtained Fast Track designation this August.
ACI-35.030 demonstrated in Phase 1b/2a clinical trials that it induces an antibody response targeting pTau while avoiding normal endogenous forms of Tau. ReTain has garnered significant interest among potential participants with prescreening rates exceeding expectations.
“The Phase 2b ReTain trial represents a potentially critical step in the battle against neurodegeneration, marking the first occasion that any active immunotherapy is being tested in the preclinical AD population. Active immunotherapies such as ACI-35.030 could provide therapeutic benefits along with improved convenience and accessibility, and the recent Fast Track designation is a significant acknowledgment of its potential value to patients,” Dr. Pfeifer commented.
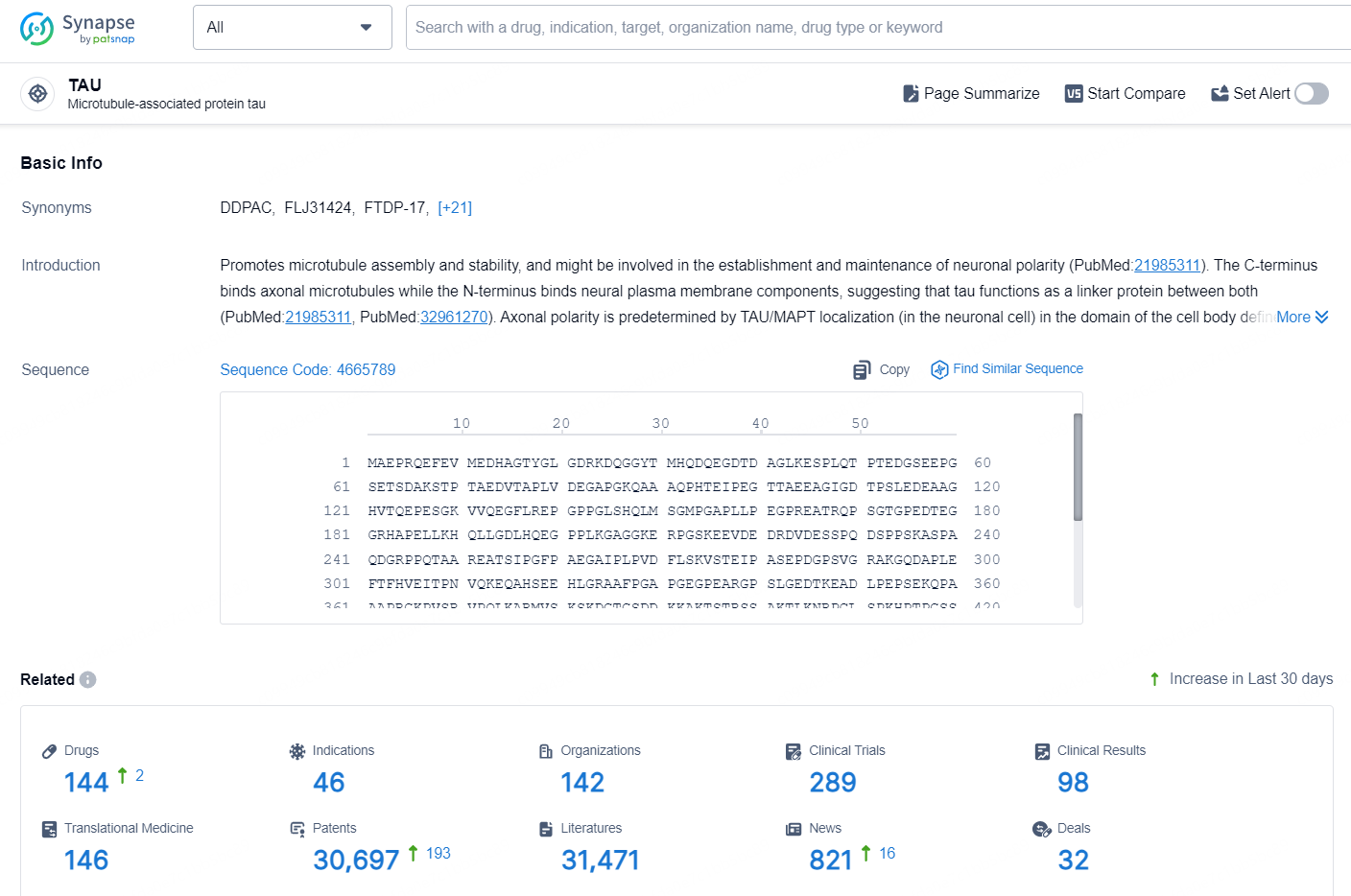
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 144 investigational drugs for the TAU target, including 46 indications, 142 R&D institutions involved, with related clinical trials reaching 289, and as many as 30697 patents.
ACI-35.030 is a prophylactic vaccine targeting TAU, developed by the pharmaceutical company AC Immune SA. The therapeutic areas of this drug include Nervous System Diseases and Other Diseases, with active indications for Alzheimer Disease and Cognitive Dysfunction. The drug has reached the highest phase of Phase 2 in its global development. It is important to note that the drug is under Fast Track regulation.