NextCure Reveals Findings from NC410 and Pembrolizumab Phase 1b Trial at ESMO 2024
NextCure, Inc. (Nasdaq: NXTC), a clinical-stage biopharmaceutical firm focused on the discovery and development of innovative, top-tier therapies for cancer treatment, has disclosed that clinical findings from the Phase 1b segment of a Phase 1b/2 trial assessing NC410, a LAIR-2 fusion protein, in conjunction with pembrolizumab were presented at ESMO 2024.
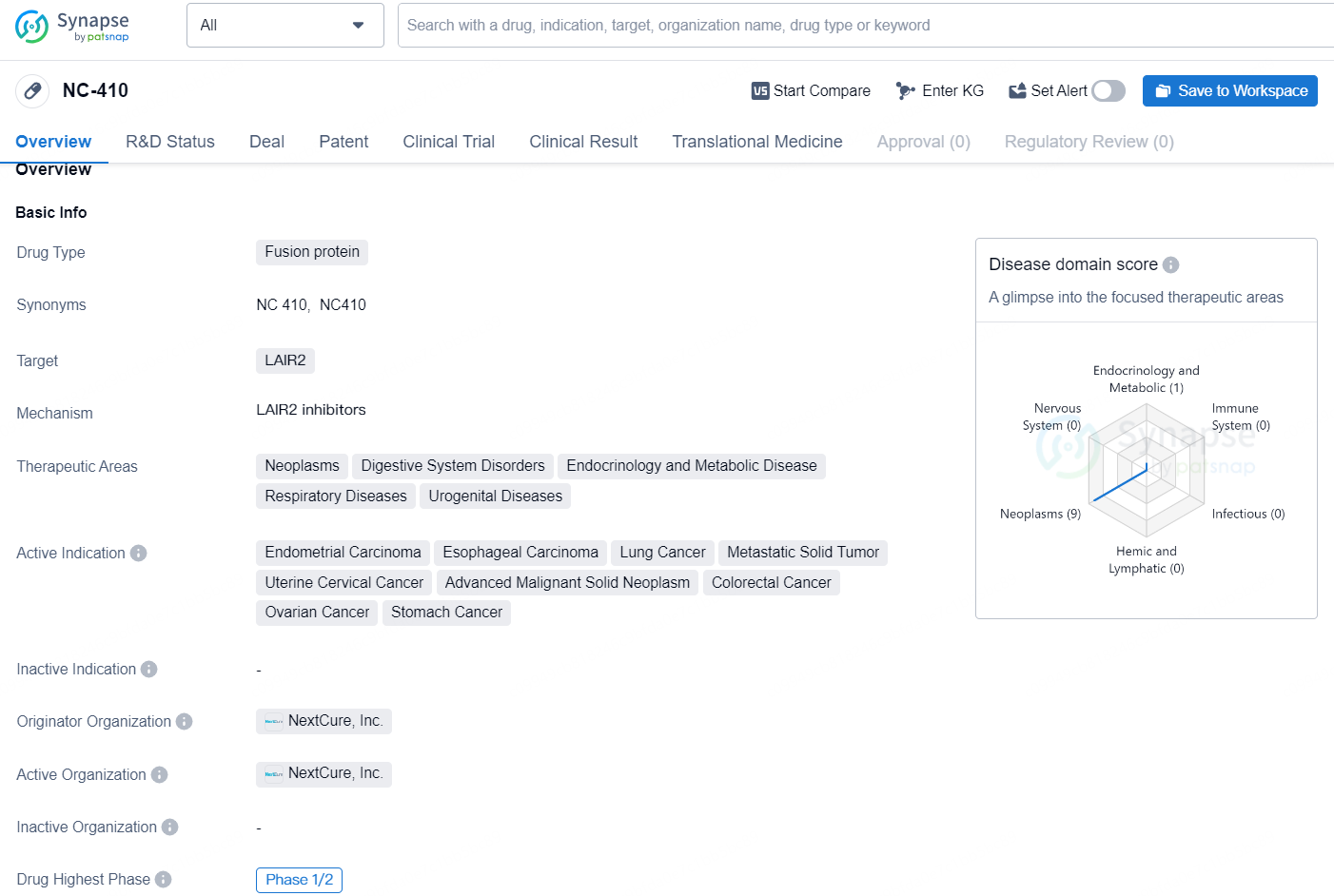
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial focusing on ovarian cancer and immune checkpoint inhibitor (ICI) naïve and refractory microsatellite stable (MSS)/microsatellite instability-low (MSI-L) colorectal cancer (CRC) has demonstrated significant clinical activity in these challenging cancer types. The findings were relayed by Dr. Emese Zsiros, M.D., Ph.D., Associate Professor of Oncology and Chair of the Department of Gynecologic Oncology at Roswell Park Comprehensive Cancer Center (ovarian), and Dr. Eric S. Christenson, M.D., Assistant Professor of Oncology at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (CRC).
"The combinatory therapy of NC410 and pembrolizumab continues to show clinical effectiveness against ovarian cancer as well as MSS/MSI-L CRC, which are particularly resistant to immunotherapy. In both cancer types, study participants who experienced partial response or stable disease showed a durability of response that is meaningful for these groups of patients," stated Dr. Udayan Guha, M.D., Ph.D., Senior Vice President, Clinical and Translational Development at NextCure. "Our team will keep monitoring the participants who are still under study and we anticipate providing an update later in the year."
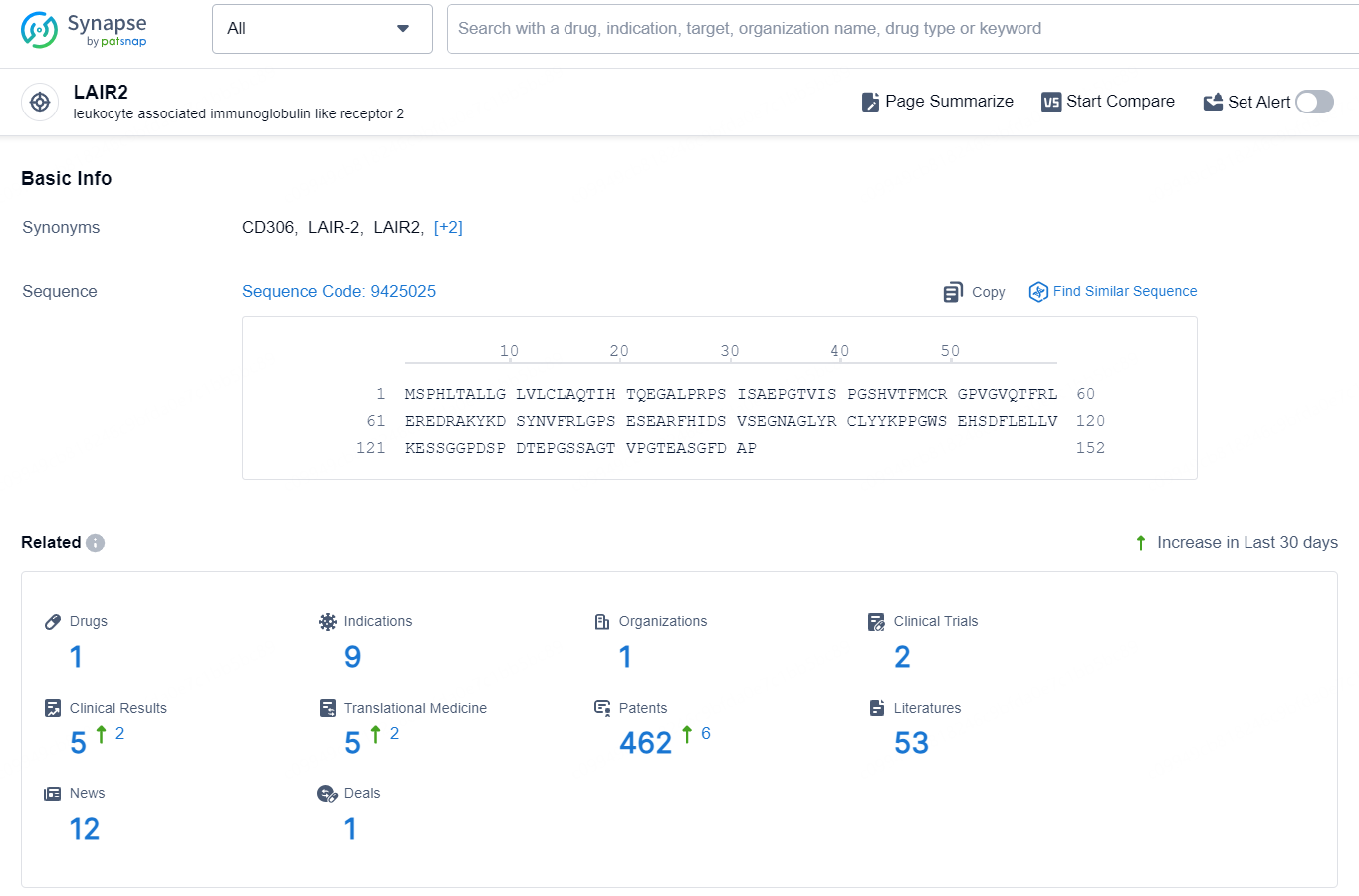
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 1 investigational drug for the LAIR2 targets, including 9 indications, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 462 patents.
NC-410 is a fusion protein drug developed by NextCure, Inc. It targets the protein LAIR2 and is being developed for the treatment of various therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic disease, respiratory diseases, and urogenital diseases. The active indications for NC-410 include endometrial carcinoma, esophageal carcinoma, lung cancer, metastatic solid tumor, uterine cervical cancer, advanced malignant solid neoplasm, colorectal cancer, ovarian cancer, and stomach cancer.