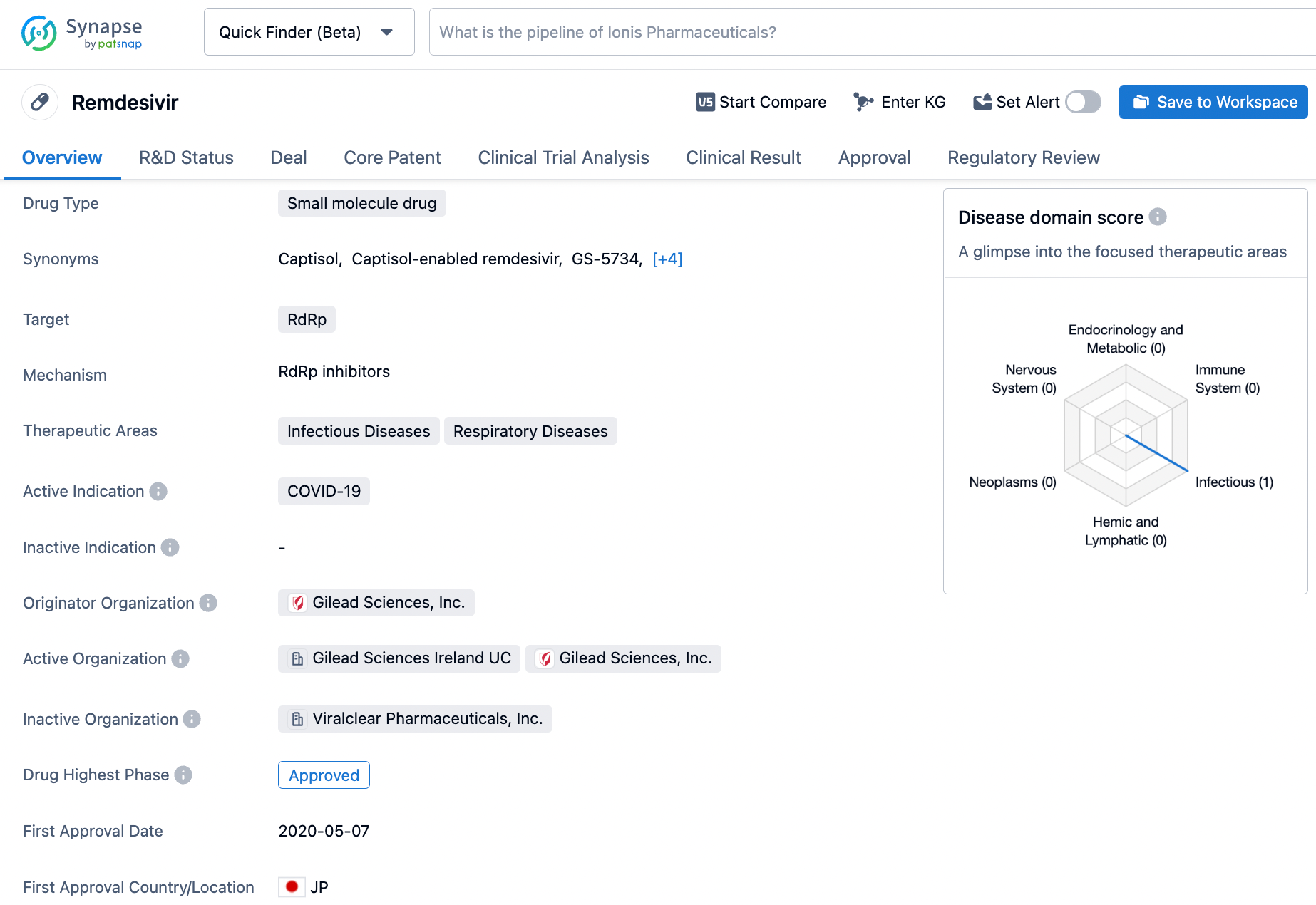
Accessing Remdesivir Information on Synapse: A Step-by-Step Approach
Remdesivir,also known by its trade name VEKLURY®,is a RNA-dependent RNA polymerase (RdRp) inhibitor developed by Gilead Sciences it was first approved by PMDA in Japan on May 7th, 2020, followed by EMA in the European Union on July 3rd, 2020, and then by FDA in US on October 22nd, 2020. indicated for the treatment of coronavirus disease 2019 (COVID-19) in adults and pediatric patients who are hospitalized or not hospitalized but at high risk for progression to severe COVID-19, including hospitalization or death, and are at least 28 days of age and weigh at least 3 kg. The drug is intended to inhibit the replication of the SARS-CoV-2 virus by interfering with its ability to make copies of itself, thus reducing the severity of the disease. Click on the image below to begin the exploration journey of Remdesivir through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!