Ankrya Therapeutics Begins Human Trials for ANK-101, a New Cancer Immunotherapy
Ankyra Therapeutics, a biotech firm at the clinical phase focusing on trailblazing tethered immunotherapies for oncology, reported the initial dosing of a participant within the dose-ranging group of a Phase 1 clinical trial. This investigation is assessing ANK-101, a cancer-specific, targeted immunotherapeutic agent for treating solid neoplasms.
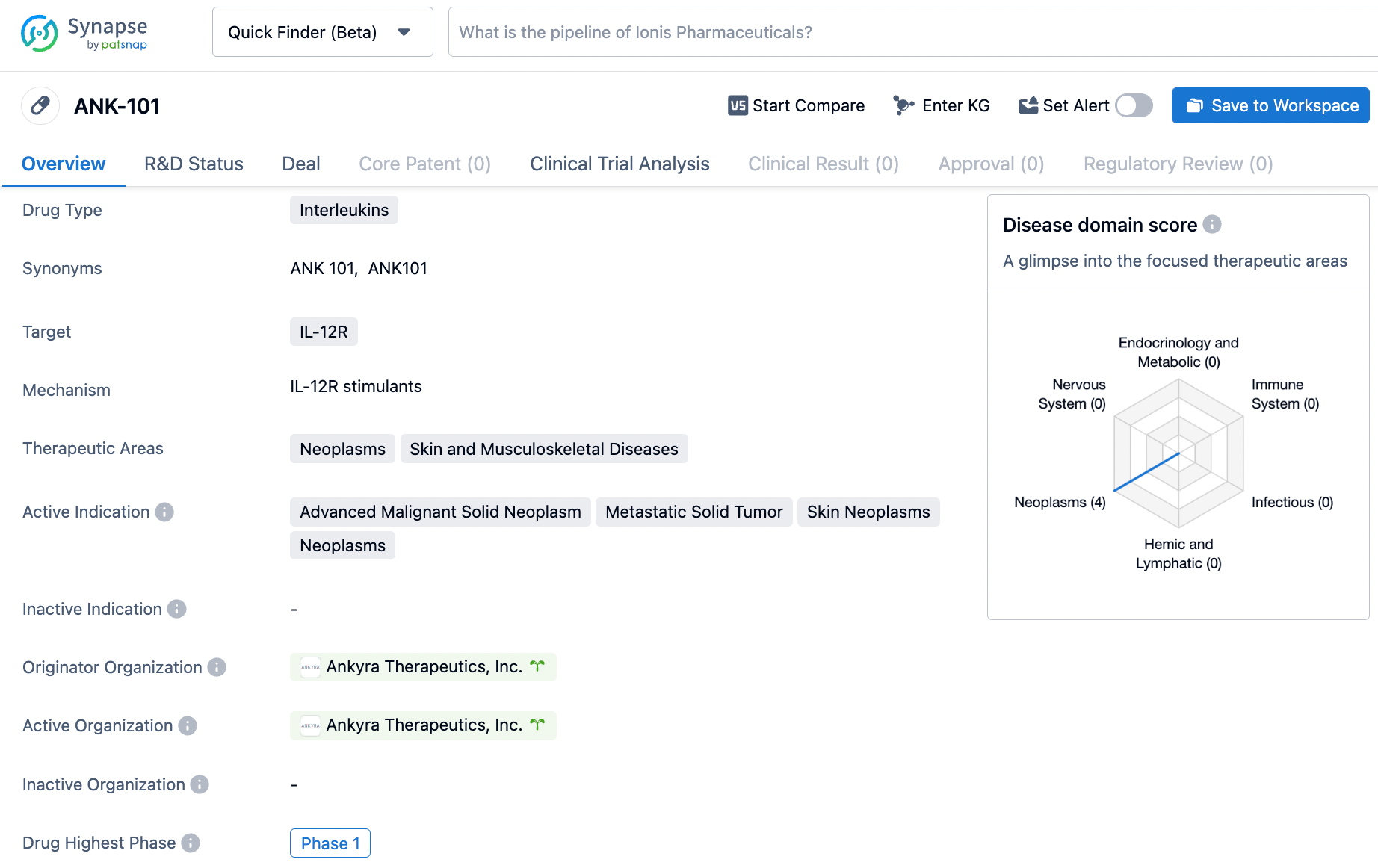
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Initiating treatment with ANK-101 in our Phase 1 solo-treatment trial signifies a critical achievement in our quest to develop a promising therapeutic option that aims to minimize adverse effects for those in need," stated Ankyra Therapeutics' Chief Executive Officer, Howard L. Kaufman, M.D. "Embarking on this endeavor is not just pivotal to the progression of our novel entity, ANK-101, but also resonates with our deep-seated pledge to deliver innovative anchored immunotherapy treatments to patients grappling with serious conditions."
ANK-101, a novel form of interleukin-12 (IL-12) amalgamated with aluminum hydroxide, is uniquely formulated for targeted delivery and prolonged presence within the tumor microenvironment. Over extended periods, it is designed to provoke and marshal immune cells that target the affected cells. ANK-101 is being evaluated in clinical trials involving patients having advanced solid tumors and who have exhibited inadequate responses to conventional therapies. ANK-101 has been shown in multiple preliminary studies to enhance immune cell infiltration into the tumors, while avoiding widespread toxic effects.
In the dose-escalation segment of the study, the tolerated levels of ANK-101 will be determined, alongside fixing a dosing schedule for a broader group of participants. The latter group will comprise around 10 patients who will receive the established dosing level. This phase targets individuals with certain types of cancer, including those affecting the skin, subcutaneous tissue, soft tissue, or lymph nodes. Secondary assessments in the study will focus on pharmacokinetics (PK), immune response, and initial efficacy of ANK-101.
"These findings will establish a clear path for the ongoing development of ANK-101 and for pinpointing the most suitable clinical applications in subsequent studies," expressed Ankyra Therapeutics' Chief Medical Officer, Joe Elassal, M.D., MBA. "Our goal is to enhance patient care with an immunotherapeutic strategy that not only has the potential to be effective but also aims at reducing the burden of treatment-related adverse effects."
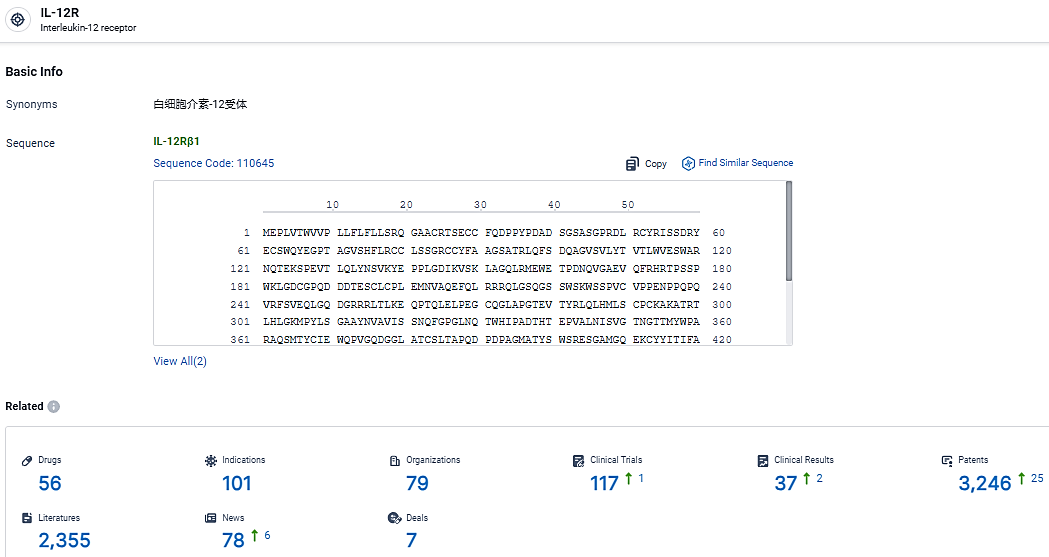
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 6, 2024, there are 56 investigational drugs for the IL-12 target, including 101 indications, 79 R&D institutions involved, with related clinical trials reaching 117, and as many as 3246 patents.
ANK-101 targets the IL-12R receptor and is being developed for the treatment of neoplasms, including advanced malignant solid neoplasms, metastatic solid tumors, and skin neoplasms. ANK-101 is currently in Phase 1 of its development, where its safety and dosage are being evaluated.