ACELYRIN, INC. Shares Encouraging Phase 3 Results for Izokibep in Hidradenitis Suppurativa
ACELYRIN, INC. (Nasdaq: SLRN), a biopharmaceutical company in the late stages of clinical development concentrating on rapid advancement and delivery of groundbreaking immunological medications, revealed that the Phase 3 trial of izokibep for Hidradenitis Suppurativa (HS) successfully met its primary endpoint of HiSCR75 at the 12-week mark. Additionally, the company announced a streamlined pipeline strategy that emphasizes lonigutamab for thyroid eye disease (TED) and is expected to prolong its cash runway.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Mina Kim, CEO of ACELYRIN, remarked, "Despite today's favorable HS data and previously disclosed results for psoriatic arthritis (PsA) paving a potential approval path for izokibep, we find that a program of this magnitude and complexity is better managed by a larger entity with the necessary resources and presence in these therapeutic areas. We are still enthusiastic about the potential of lonigutamab to meet the unaddressed needs of TED patients.
In line with our disciplined capital allocation strategy, we have decided to prioritize advancing lonigutamab through the later stages of development using our current cash reserves. Together with a workforce reduction, this strategic pivot enables us to extend our financial runway to mid-2027 and fully fund both Phase 3 clinical trials for lonigutamab."
In the international Phase 3 study for HS, izokibep elicited statistically significant responses across various efficacy metrics at 12 weeks. Specifically, 33% of patients administered 160mg of izokibep weekly (QW) achieved HiSCR75, versus 21% for the placebo group (p-value=0.0294). Higher-tier endpoints showed that 25% of patients reached HiSCR90, compared to 9% on placebo (p-value=0.0009), and 22% reached HiSCR100, compared to 8% on placebo (p-value=0.001).
Although the primary endpoint assessment was at 12 weeks, ACELYRIN has continued the placebo-controlled dosing through week 16. Data from two-thirds of the patients at this point indicates a progressing enhancement in HiSCR responses over time.
No novel safety issues were seen for izokibep. The most frequent adverse events were mild-to-moderate injection site reactions, headache, nasopharyngitis, fatigue, and diarrhea. Importantly, there were no reports of candida infection, liver toxicity, or suicidal ideation/behavior among those treated with izokibep.
ACELYRIN plans to finalize the ongoing PsA and HS trials but will halt new investments in these indications. The ongoing Phase 2b/3 study of izokibep in uveitis will continue up to the primary endpoint, with top-line data anticipated in the fourth quarter of 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
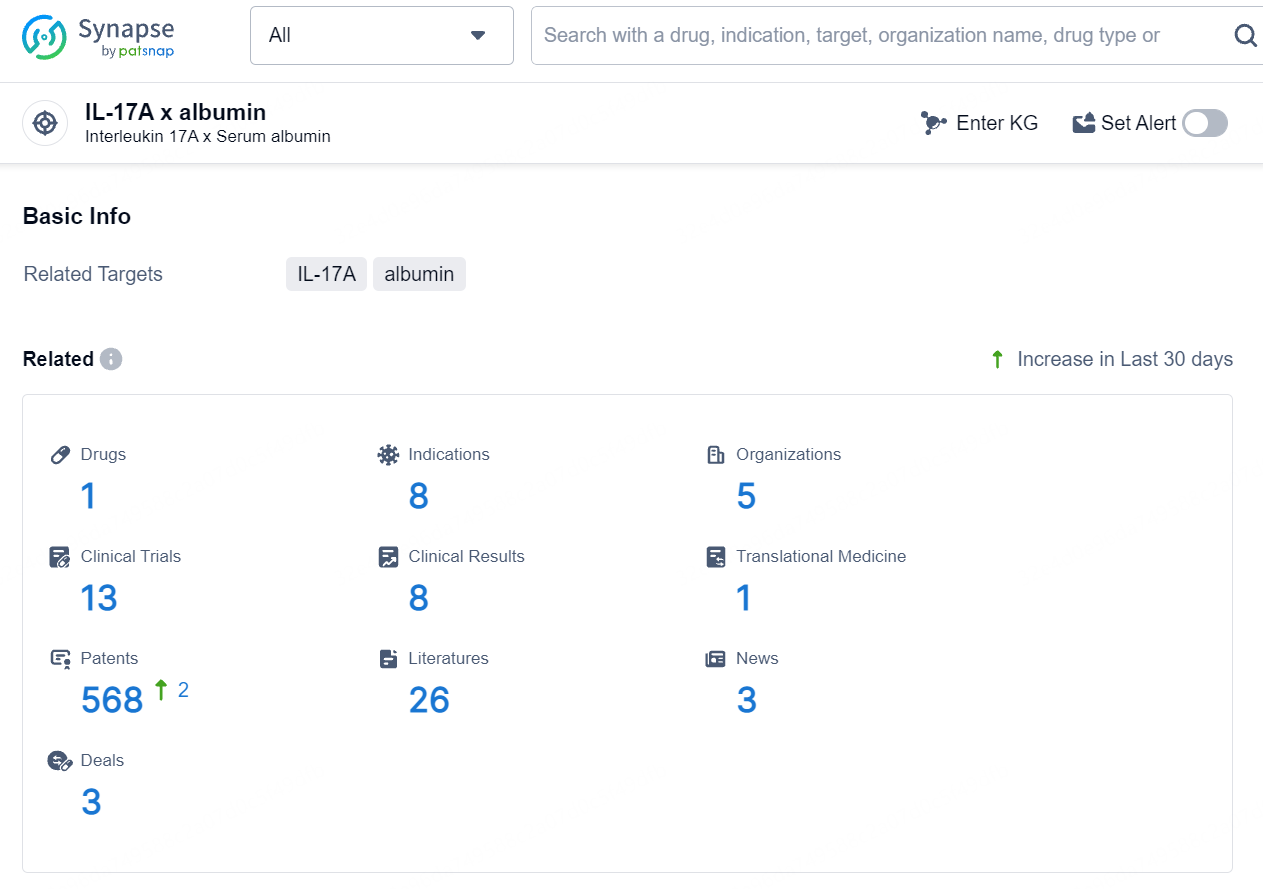
According to the data provided by the Synapse Database, As of August 15, 2024, there are 1 investigational drug for the IL-17A x albumin target, including 8 indications, 5 R&D institutions involved, with related clinical trials reaching 13, and as many as 568 patents.
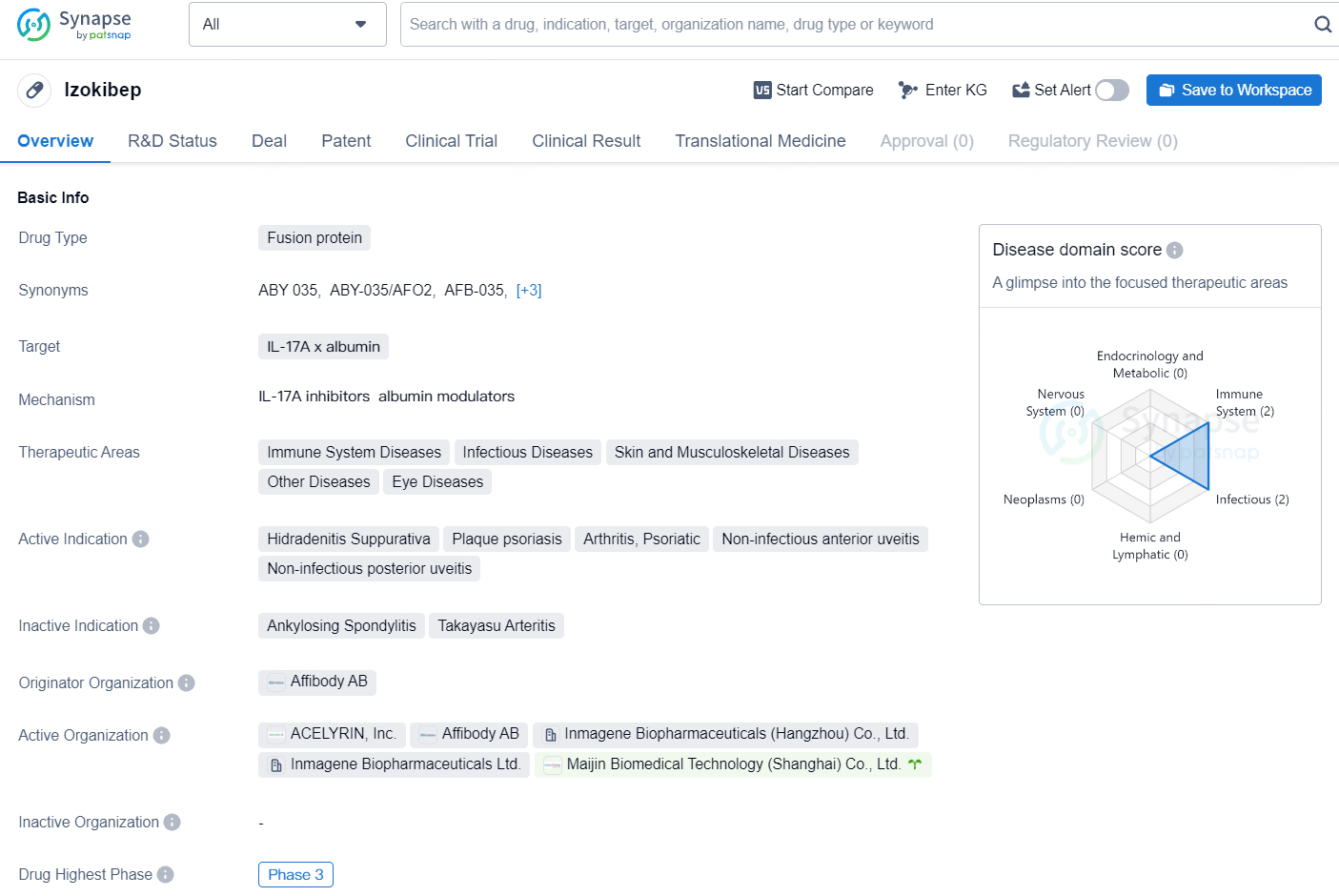
Izokibep is a fusion protein drug developed by Affibody AB, targeting IL-17A x albumin. The drug falls under the therapeutic areas of Immune System Diseases, Infectious Diseases, Skin and Musculoskeletal Diseases, Other Diseases, and Eye Diseases. The active indications for Izokibep include Hidradenitis Suppurativa, Plaque psoriasis, Arthritis, Psoriatic, Non-infectious anterior uveitis, and Non-infectious posterior uveitis. Currently, the drug has reached the highest phase of development, Phase 3, both globally and in China.