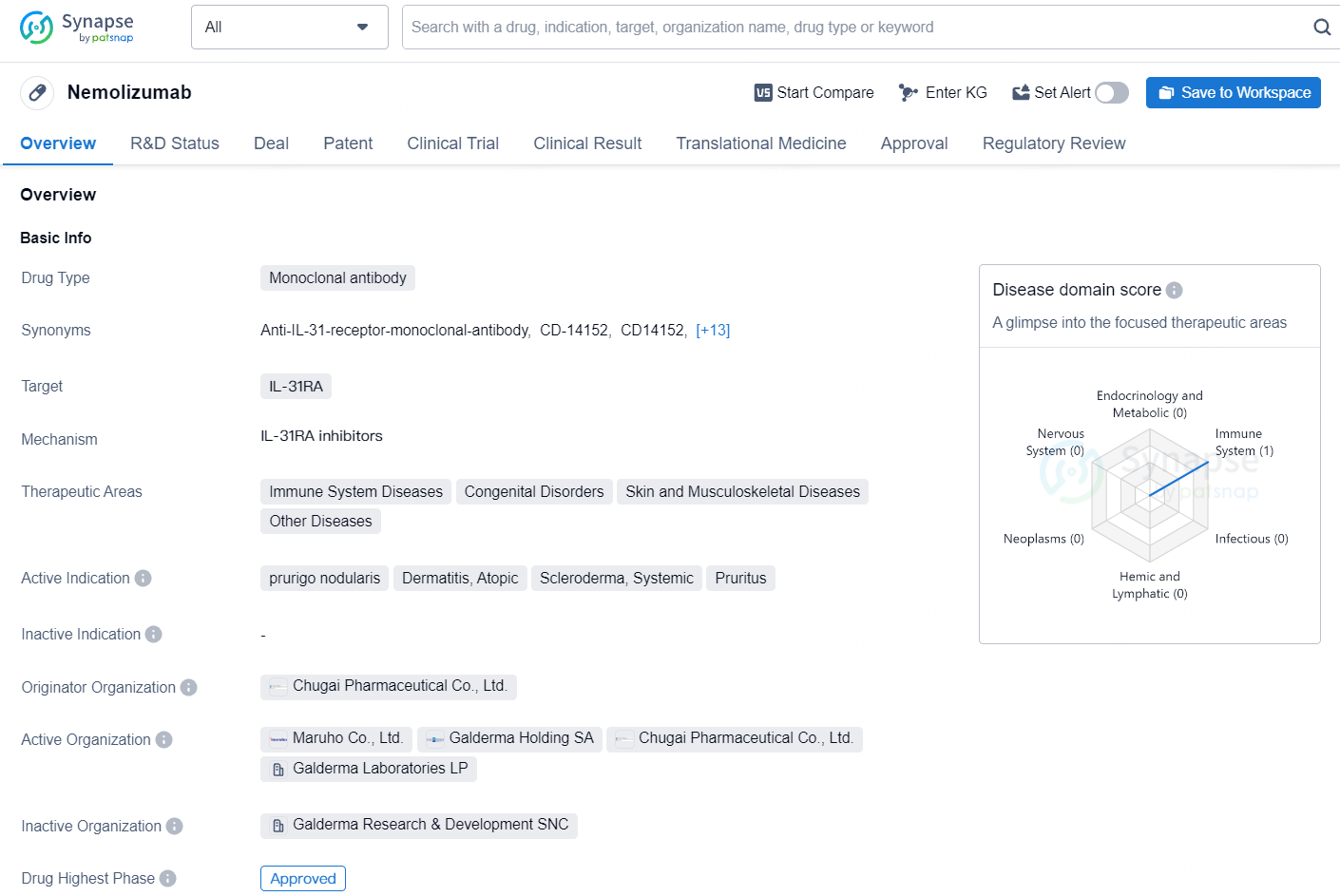
Galderma Gets U.S. FDA Nod for Nemluvio® (nemolizumab) to Treat Adult Prurigo Nodularis
Galderma has revealed that the U.S. Food and Drug Administration (FDA) has given the green light to Nemluvio® (nemolizumab) as a pre-filled pen designed for subcutaneous injection to treat adults suffering from prurigo nodularis.1 In December 2019, Nemluvio received Breakthrough Therapy Designation, and in February 2024, it was assigned Priority Review by the U.S. FDA—a classification meant for drugs that could greatly enhance treatment methods for severe medical conditions.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Prurigo nodularis is a frequently underdiagnosed neuroimmune dermatological condition that is thought to impact up to 181,000 individuals in the United States. This ailment presents with a range of incapacitating symptoms, such as persistent itching, nodules on the skin over extensive areas, and disrupted sleep. The considerable impact on patients underscores the necessity for alternative therapies that can effectively alleviate the primary symptoms and signs of the disease. Nemluvio works by specifically inhibiting IL-31 cytokine signaling, which plays a key role in inducing itch and is implicated in inflammation, compromised epidermal differentiation, and fibrosis in prurigo nodularis.
This drug's approval stems from successful outcomes in the phase III OLYMPIA clinical trials—the most extensive trial program ever conducted for prurigo nodularis—where Nemluvio showed marked and clinically significant improvements in itching and skin nodules by the 16th week, with noticeable itch reduction as early as the 4th week.
The phase III OLYMPIA 1 and OLYMPIA 2 trials investigated the efficacy and safety of subcutaneous Nemluvio administered every four weeks in over 500 prurigo nodularis patients. Both trials met their primary and key secondary endpoints, with findings as follows:
In OLYMPIA 1 and 2, 56% and 49% of Nemluvio-treated participants, respectively, saw at least a four-point decline in itch intensity at Week 16, compared to 16% in both placebo groups (primary endpoint).
At Week 4, 41% of Nemluvio-treated patients in OLYMPIA 1 and 2 achieved a similar reduction in itch intensity, compared to 6% and 7% in the placebo group (key secondary endpoint).
By Week 16, 26% and 38% of Nemluvio-treated individuals in OLYMPIA 1 and 2, respectively, reached almost or complete clearance of skin nodules, as per the IGA score, compared to 7% and 11% in the placebo group (primary endpoint).
At Week 16, 50% and 52% of Nemluvio-treated patients in OLYMPIA 1 and 2, respectively, had at least a four-point reduction in sleep disturbance, compared to 12% and 21% in the placebo group
The trials also met other key secondary goals, confirming rapid alleviation of itch and sleep disturbance associated with prurigo nodularis within four weeks of treatment commencement. Nemluvio had a generally favorable safety and tolerance profile, consistent with results from the phase II trial and similar between the OLYMPIA 1 and 2 trials.
Additionally, the U.S. FDA has accepted Nemluvio's Biologics License Application for treating moderate-to-severe atopic dermatitis, with a regulatory decision expected later this year. Concurrently, Galderma's marketing authorization requests for Nemluvio in prurigo nodularis and atopic dermatitis are under review by various regulatory bodies, including the European Medicines Agency and Health Canada. Similar reviews are underway in Australia, Singapore, Switzerland, and the United Kingdom through the Access Consortium. Further submissions to additional regulatory authorities are planned through 2024.
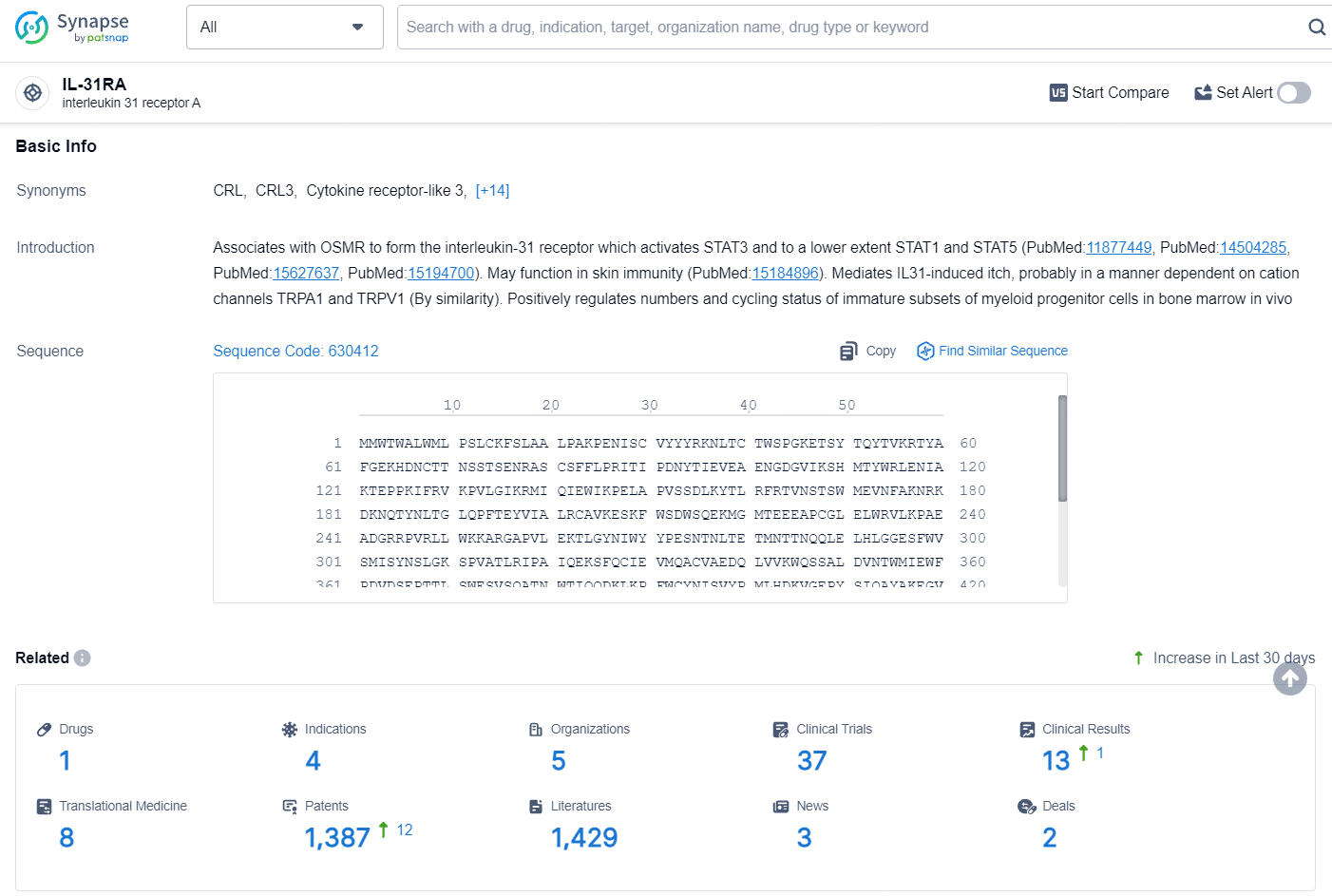
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 15, 2024, there are 1 investigational drug for the IL-31RA target, including 4 indications, 5 R&D institutions involved, with related clinical trials reaching 37, and as many as 1387 patents.
Nemolizumab is a monoclonal antibody drug that targets IL-31RA and is used in the treatment of various immune system diseases, congenital disorders, skin and musculoskeletal diseases, and other related conditions. The drug's active indications include prurigo nodularis, atopic dermatitis, systemic scleroderma, and pruritus.