Aclaris Pharma Shares Initial Outcomes after a Month-Long Phase 2b Study on ATI-1777 in Treating Mild to Intense Eczema
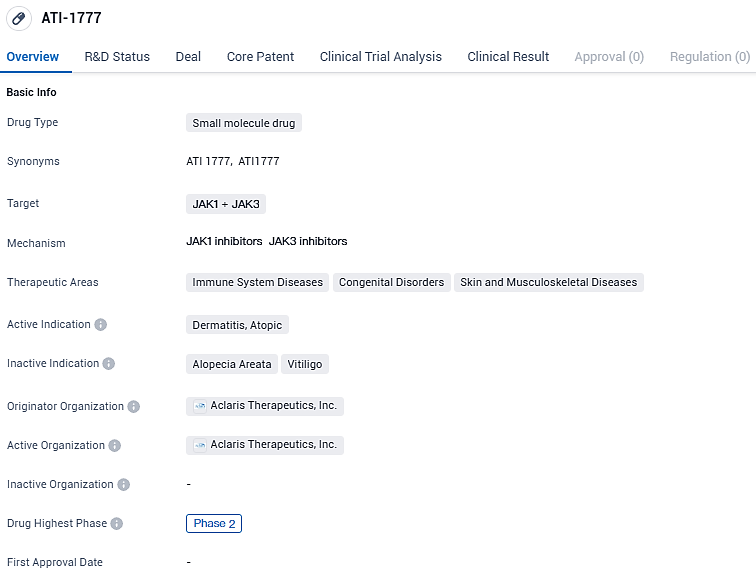
Aclaris Therapeutics, Inc., a biopharmaceutical entity engaged in the clinical development stage, dedicated to crafting innovative therapeutic agents to combat immuno-inflammatory disorders, disclosed preliminary outcomes from a Phase 2b clinical trial. This trial investigated the effectiveness of ATI-1777, a pioneering "soft"JAK 1/3 inhibitor designed for topical application, in participants suffering from atopic dermatitis ranging from mild to severe intensity. The drug candidate ATI-1777 originated from the company's own KINect® drug discovery technology.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The ATI-1777-AD-202 study represented a stage 2b clinical trial conducted in multiple centers, featuring a randomized, double-blind, vehicle-controlled, and parallel-group design. This research aimed to assess the efficacy, safety, tolerability, and pharmacokinetics (PK) profiles of ATI-1777 applied twice a day at varying concentrations (0.5%, 1%, and 2%), alongside a comparison to a single 2% concentration used once daily.
ATI-1777 was specifically crafted into a spray that contained an emollient for the purposes of this investigation. Around 250 subjects with atopic dermatitis (AD) spanning mild to severe levels were enrolled. The demographic included adults and youngsters starting at 12 years of age. These participants were distributed across 30 research locations throughout the United States.
Dr. Neal Walker, head of Aclaris' Board, expressed, "ATI-1777 has shown encouraging rates of therapeutic response that align with those of current treatments in the market. Moreover, its safety profile appears to be favorably distinct compared to JAK inhibitors, highlighting its minimal systemic absorption as observed in the two Phase 2 trials. The ease of use provided by the spray form, combined with the prospects of reducing application to once daily, may present notable benefits for individuals dealing with atopic dermatitis."
Douglas Manion, M.D., the CEO of Aclaris, acknowledged the contributions of the trial participants and lauded the commitment of the company's staff in conducting the research. He added, "In line with our prior declarations, our objective is to forge a partnership for the development and commercialization of this particular drug program. This might expand to treating other conditions, with vitiligo as a possible example."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
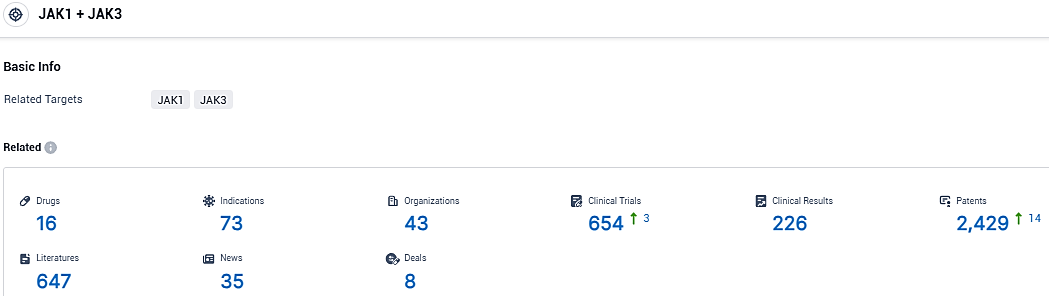
According to the data provided by the Synapse Database, As of January 16, 2024, there are 16 investigational drugs for the JAK 1/3 target, including 73 indications, 43 R&D institutions involved, with related clinical trials reaching 654, and as many as 2429 patents.
ATI-1777 targets JAK1 and JAK3 and is being investigated for its potential in treating immune system diseases, congenital disorders, and skin and musculoskeletal diseases, specifically dermatitis, atopic. Currently in Phase 2, ATI-1777 has shown promising results in early clinical trials and may progress to Phase 3 trials in the future.