ACR 2023: Amgen unveils fresh results from second phase study of dazodalibep for Sjögren's disorder
Amgen has revealed fresh information from the Phase 2 trial of dazodalibep, an experimental drug aimed to treat Sjögren's. The findings will be showcased in discussions at the American College of Rheumatology Convergence 2023 taking place from November 10-15 in San Diego. The study's results indicate that dazodalibep could potentially lessen both the systemic and symptomatic disease impacts for two separate patient groups.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
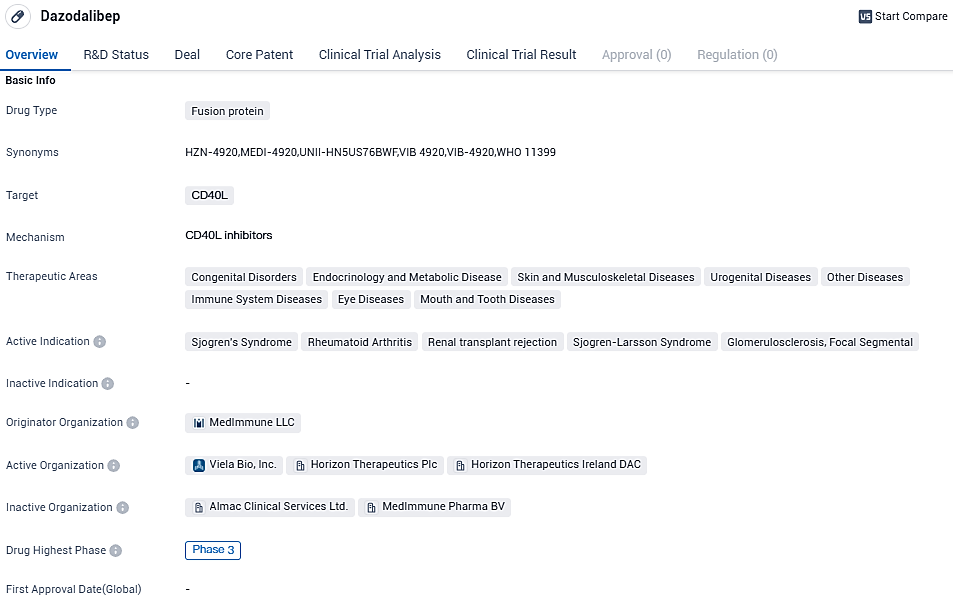 The clinical development of dazodalibep, a CD40 ligand antagonist, underwent Phase 2 of its research. It was trialed in a double-blind, placebo-controlled, randomized crossover investigation, evaluating two demographic groups of Sjögren's syndrome: individuals with moderate to extreme systemic activity and those expressing equivalent symptom severity, despite not having extra organ engagement.
The clinical development of dazodalibep, a CD40 ligand antagonist, underwent Phase 2 of its research. It was trialed in a double-blind, placebo-controlled, randomized crossover investigation, evaluating two demographic groups of Sjögren's syndrome: individuals with moderate to extreme systemic activity and those expressing equivalent symptom severity, despite not having extra organ engagement.
By May 2023, updates provided at the 2023 EULAR Congress stated that as of Day 169, dazodalibep treatment was successful in reaching the primary goal of the study in both patient clusters. The switch reports, as highlighted in the ACR presentations, showed that by Day 169, patients who initially received dazodalibep were given placebo treatment, and those who started with placebo were switched to dazodalibep. Patients were monitored for safety reasons for an additional 3 months post last dose administration.
According to Dr. David M. Reese, executive vice president of Research and Development at Amgen, there haven't been any FDA-approved treatments for Sjögren's syndrome designed to adjust the progression of the disease so far. However, the promising results of the Phase 2 trial of dazodalibep indicate that it may tackle root of the problem by minimizing systemic disease activity and alleviating severe symptoms such as fatigue and dryness.
Dazodalibep operates as a CD40 ligand antagonist, hindering the association between T cells and CD40-displaying B cells, thereby preventing the overstimulation of the CD40 ligand co-stimulatory pathway. This pathway's overactivity is linked to various autoimmune diseases. Amgen has announced that further exploration of dazodalibep will also take place in relation to focal segmental glomerulosclerosis, a rare kidney condition marked by glomeruli scarring.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
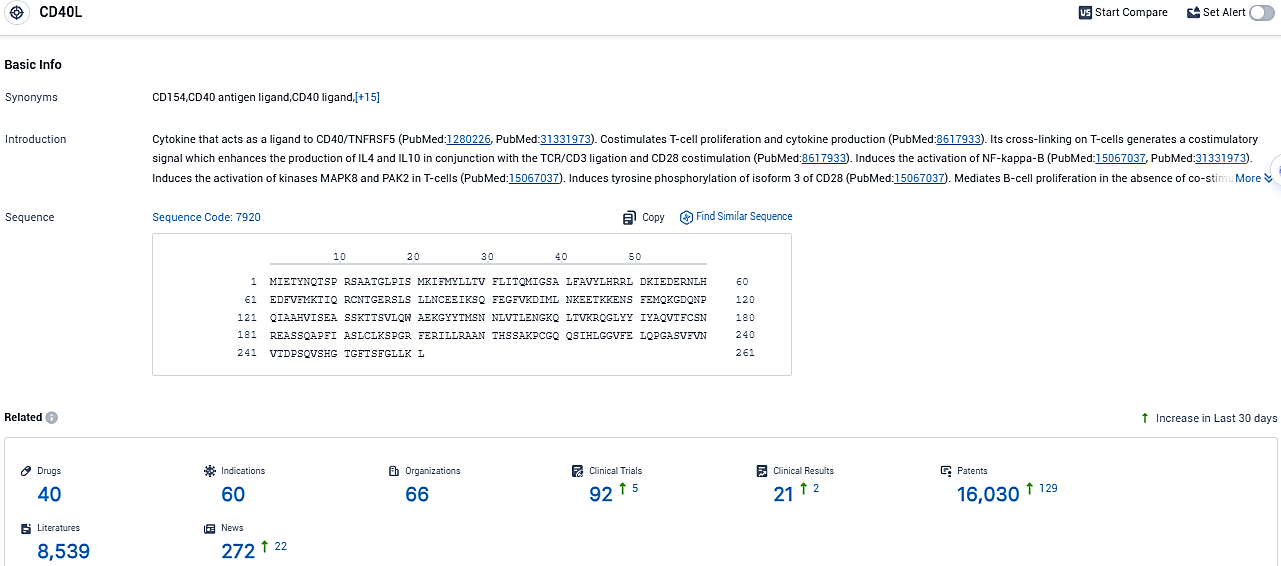
According to the data provided by the Synapse Database, As of November 14, 2023, there are 40 investigational drugs for the CD40L target, including 60 indications, 66 R&D institutions involved, with related clinical trials reaching 92, and as many as 16030 patents.
Dazodalibep shows promise as a potential treatment for a wide range of diseases across various therapeutic areas.The discontinuation of its development in China suggests that there may be specific challenges or regulatory issues in that market. Further research and development, as well as regulatory approvals, will be necessary to determine the drug's ultimate success and availability to patients.


![[177Lu]Lu-PSMA-617: Brief Review of its R&D progress and the clinical outcome in 2023 ESMO](https://synapse-static-patsnap-com.libproxy1.nus.edu.sg/strapi-static/Blog_cover_18_df71aab65d.jpg)

