Acrotech Biopharma and Evive Biotech have obtained FDA approval for Ryzneuta® to treat chemotherapy-induced Neutropenia (CIN)
On 16th November 2023, the FDA gave the green light to Ryzneuta® (Efbemalenograstim alfa), a treatment purposed to mitigate the possibility of infection, specifically febrile neutropenia, in mature individuals dealing with non-myeloid malignancies. This approval was declared by Evive Biotech in collaboration with Acrotech Biopharma, noting that the specified patients would be following a course of myelosuppressive anti-cancer medication, with a notable occurrence of febrile neutropenia.
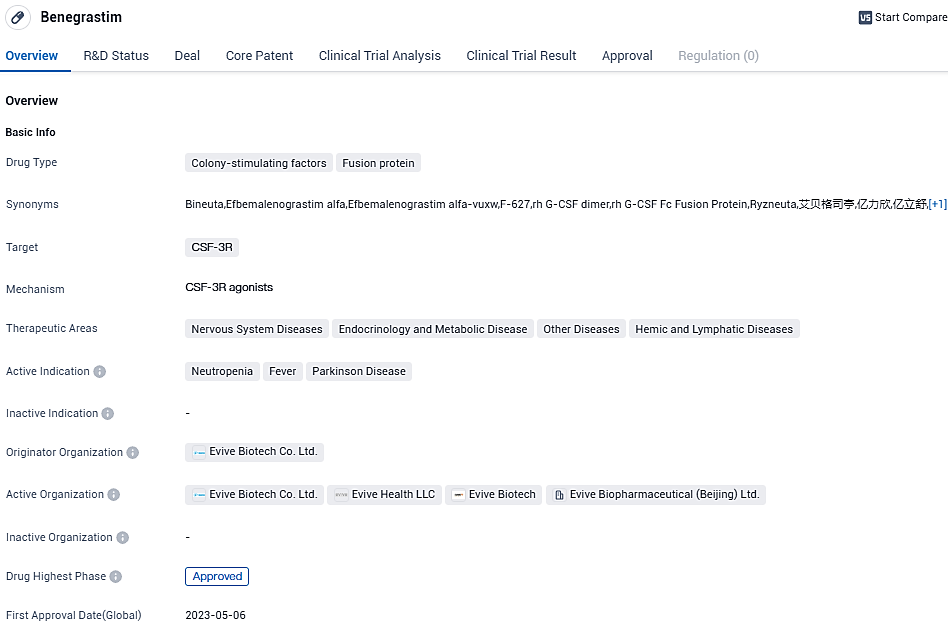
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The endorsement stems from findings of two integral Phase 3 Studies, GC-627-04 and GC-627-05, conducted in the United States and Europe. Ryzneuta® functions as a unique, long-acting Granulocyte colony-stimulating factor (G-CSF), responsible for promoting the growth, diversification, and dispatch of neutrophil progenitors. It enhances the immune capacity of cancer sufferers and counteracts the adverse effects of chemotherapy-induced neutropenia.
The multi-center, randomized, multiple-dose, active-controlled Study GC-627-05 was essentially designed to contrast the efficacy and safety of Ryzneuta® and Neulasta® (Pegfilgrastim). The trial successfully accomplished its primary and secondary objectives regarding efficacy and safety.
Typically, neutropenia is a prevalent chemotherapy side effect, illustrated by chronically low neutrophil counts due to the application of chemotherapy and other anti-cancer medications. This increases the chance of unwanted events such as infections and fever in cancer patients undergoing chemotherapy.
Ryzneuta® is a novel dimetric G-CSF long-acting fusion protein void of PEGylation or Tween-80. Ryzneuta®'s distinct molecular structure may result in enhanced G-CSF receptor activation capabilities, hence bypassing issues potentially brought about by PEG or Tween-80.
As quoted by Simon Li, M.D., Ph.D., CEO & CMO of Evive, "Ryzneuta® represents the first biologics independently created by Evive Biotech, and its endorsement testifies to Evive R&D team's potential for independent global development of innovative biologics". He added, "We are excited about partnering with Acrotech to offer this novel treatment to more CIN sufferers in the US."
Ryzneuta® bolsters the immune system's defense against infection by elevating the production of neutrophils, thwarting possible chemotherapy dose downsizing and interruptions which could undermine treatment results. Progressively, Ryzneuta® anticipates further regulatory authorizations, positioning Ryzneuta® to offer urgently needed, efficacious, first-line treatment and alternative therapy to global cancer patients.
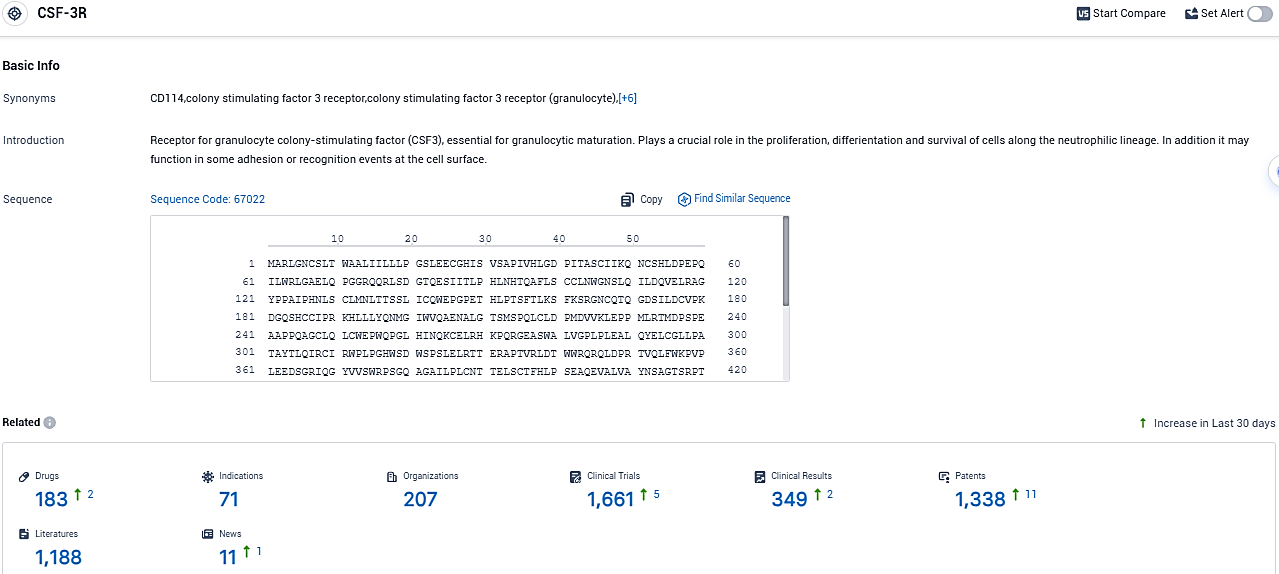
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 30, 2023, there are 183 investigational drugs for the CSF-3R target, including 71 indications, 207 R&D institutions involved, with related clinical trials reaching 1661, and as many as 1338 patents.
Ryzneuta® (Efbemalenograstim alfa) is developed for the treatment of Chemotherapy-Induced Neutropenia in cancer patients after chemotherapy. In May this year, Ryzneuta® was approved and launched in China. In addition, the facility producing Ryzneuta® has successfully passed the on-site GMP inspections conducted by ANVISA and EMA.