Adcentrx Therapeutics Gains China's NMPA IND Approval for ADRX-0706, a Nectin-4 Antibody-Drug Conjugate for Advanced Solid Tumors
Adcentrx Therapeutics, an innovator in the field of biotechnology focusing on the development of Antibody-Drug Conjugate therapies for cancer and critical illnesses, revealed that the China National Medical Products Administration has approved its Investigational New Drug application for ADRX-0706. This approval permits the inclusion of clinical centers in China in its currently active Phase 1a/1b trial aimed at treating certain advanced solid tumors.
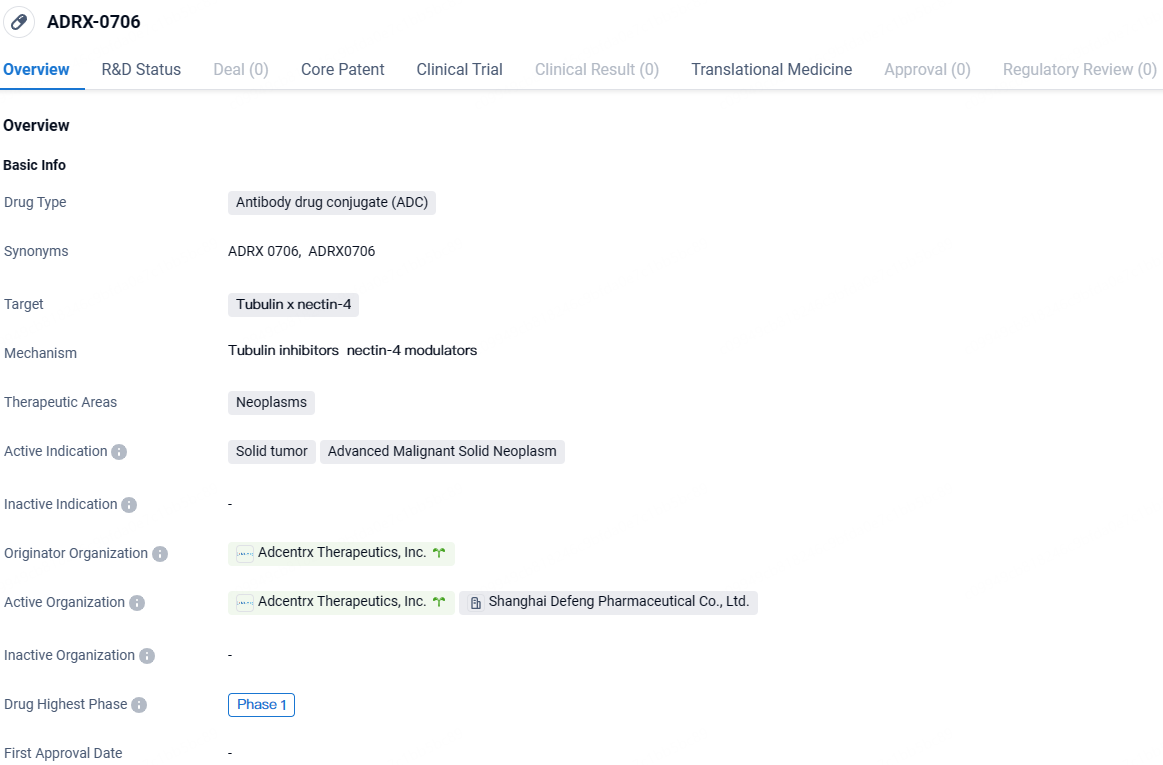
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ADRX-0706 consists of an advanced ADC that features a new human IgG1 antibody aimed at human Nectin-4, connected to the unique tubulin inhibitor AP052 via the cutting-edge i-Conjugation™ technology of Adcentrx. This technology employs a cleavable linker and robust conjugation chemistry. The innovative nature of this platform allows the creation of a stable ADC with a drug-antibody ratio of eight, showing a greatly enhanced therapeutic potential as evidenced in preclinical evaluations.
Nectin-4 has been recognized as an effective target for ADC therapies because it is highly expressed in several solid cancers while having minimal presence in healthy tissues. Its significant role in promoting tumor growth and its links to negative outcomes and resistance to traditional treatments have been well documented.
Regarding the ADRX-0706 IND approval by NMPA, Hui Li, Ph.D., the Founder and CEO of Adcentrx, expressed the importance of this event, stating, “This clearance gives us the leverage to recruit patients from both the U.S. and China, helping us gather crucial data across various patient demographics. This data collection will be pivotal in advancing ADRX-0706 for treating various cancers with significant unmet needs.”
The initial human Phase 1a/b clinical trial for ADRX-0706 involves both a dose-escalation and dose-expansion phase and is being conducted across multiple centers as an open-label study. The focus of the trial, which targets patients with specific advanced solid tumors, is to establish the safety, tolerability, and ideal dosing of ADRX-0706. Initial findings from this trial are anticipated by mid-2024.
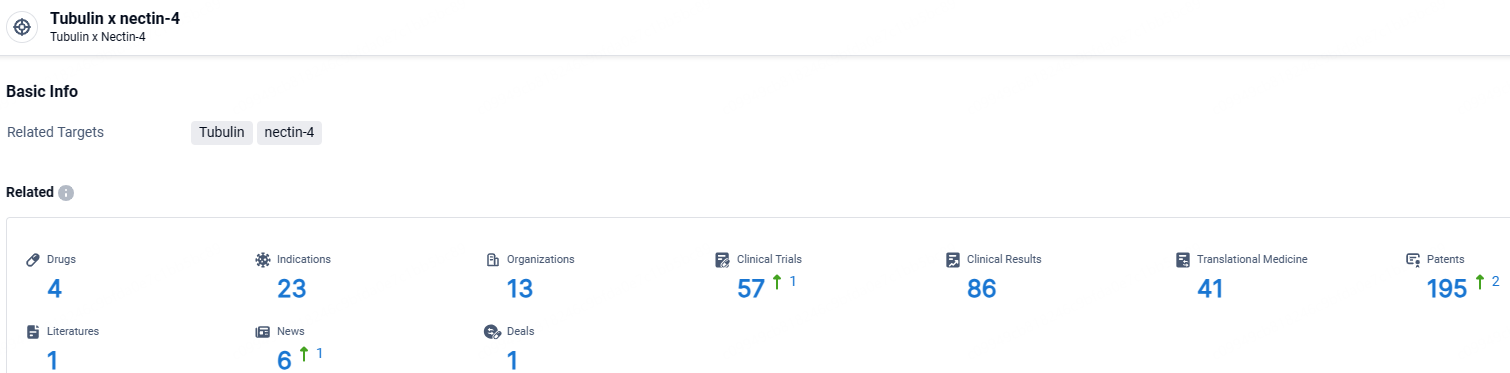
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 4 investigational drugs for the tubulin and nectin-4 target, including 23 indications, 13 R&D institutions involved, with related clinical trials reaching 57, and as many as 195 patents.
ADRX-0706 targets tubulin and nectin-4 for the treatment of neoplasms, specifically solid tumors and advanced malignant solid neoplasms. The drug is currently in Phase 1 of clinical development and has received IND approval in China. Further research and clinical trials will be needed to determine the safety and efficacy of ADRX-0706 in treating these conditions.