Adicet Bio Begins Dosing First Lupus Nephritis Patient in ADI-001 Phase 1 Trial
Adicet Bio, Inc. (Nasdaq: ACET), a biotechnology firm in the clinical phase dedicated to discovering and creating allogeneic gamma delta T cell treatments for autoimmune conditions and cancer, has announced that dosing has begun for the initial LN patient in its Phase 1 clinical study assessing ADI-001 for autoimmune diseases.
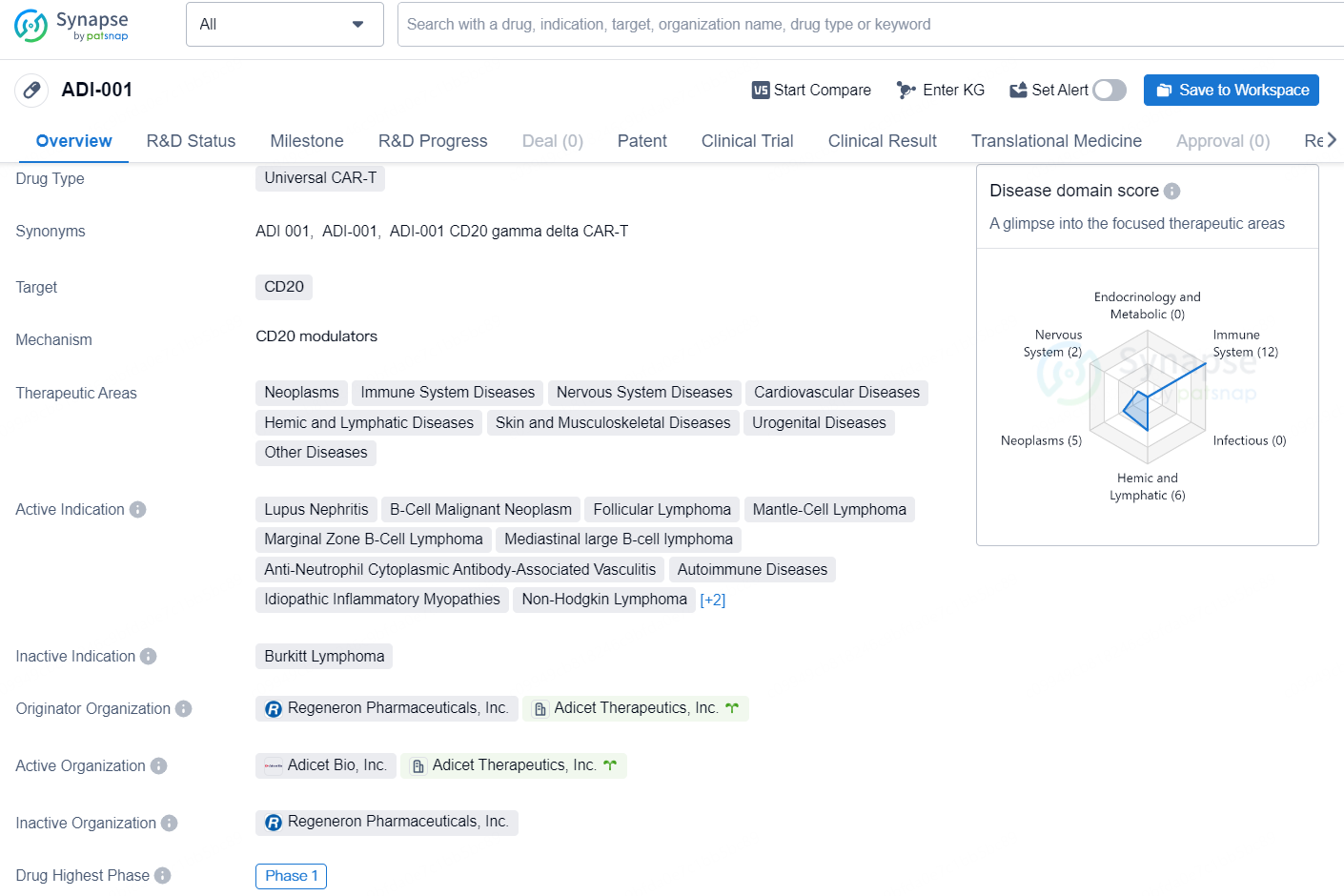
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Dosing the first patient with lupus nephritis in our Phase 1 trial for ADI-001 represents a significant advancement in our goal of enhancing the quality of life for individuals suffering from autoimmune disorders, notably lupus nephritis," stated Francesco Galimi, M.D., Ph.D., Senior Vice President and Chief Medical Officer at Adicet Bio. "Our clinical biomarker findings from the study on non-Hodgkin’s lymphoma showcase strong tissue trafficking and complete depletion of CD19+ B cells in the peripheral blood and secondary lymphoid tissues, indicating that ADI-001 could serve as a groundbreaking off-the-shelf treatment alternative for multiple autoimmune conditions. Furthermore, the FDA's Fast Track Designation for ADI-001 in relapsed/refractory class III or class IV LN, along with the approval of our IND amendment for ADI-001 targeting SPS and IIM, underscores the urgent and widespread demand for available therapies to tackle autoimmune diseases."
Dr. Galimi added, "With clinical sites now open for participant recruitment and more anticipated to launch soon, we expect to present initial clinical results from the trial in the first half of 2025. Additionally, we are eager to begin recruiting patients with SLE, SSc, IIM, and SPS in the first quarter of 2025, followed by those with AAV in the latter half of 2025."
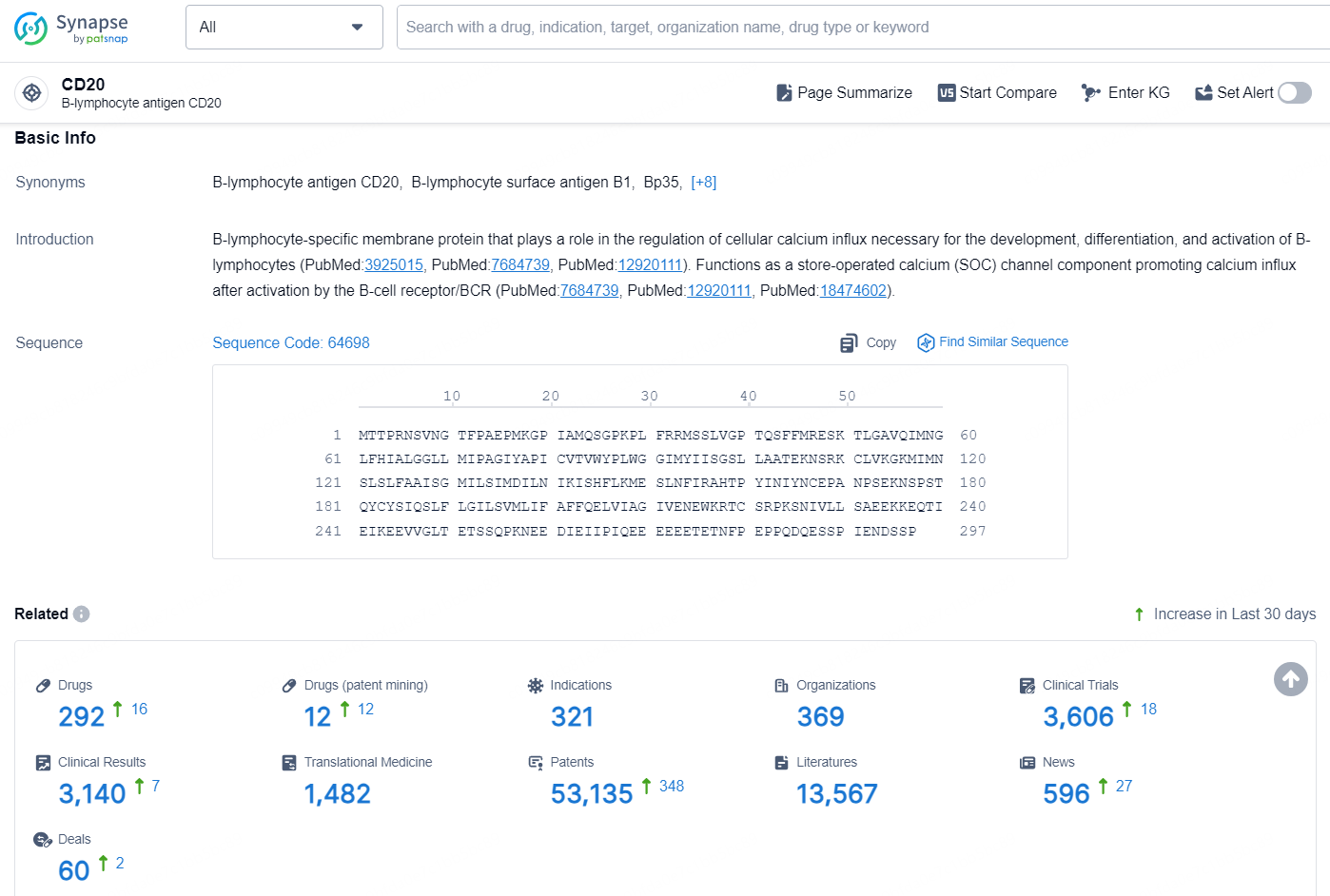
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 292 investigational drugs for the CD20 target, including 321 indications, 369 R&D institutions involved, with related clinical trials reaching 3606, and as many as 53135 patents.
ADI-001 is a Universal CAR-T drug targeting CD20, with potential therapeutic applications in a wide range of disease areas including neoplasms, immune system diseases, nervous system diseases, cardiovascular diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.