Seismic Therapeutics Presents Preclinical Findings on Dual-Target Antibody S-4321 for Autoimmunity at ACR 2024
Seismic Therapeutic, Inc., a company focused on machine learning in immunology, has announced that it will present new findings for S-4321, its next-generation PD-1: FcγRIIb-selective bifunctional agonist antibody aimed at treating autoimmune disorders. This presentation will occur at ACR Convergence 2024, the annual gathering of the American College of Rheumatology, scheduled for November 14-19, 2024, in Washington, DC. Following encouraging preclinical data, Seismic intends to launch a Phase 1 clinical trial of S-4321 involving healthy volunteers in the early part of 2025.
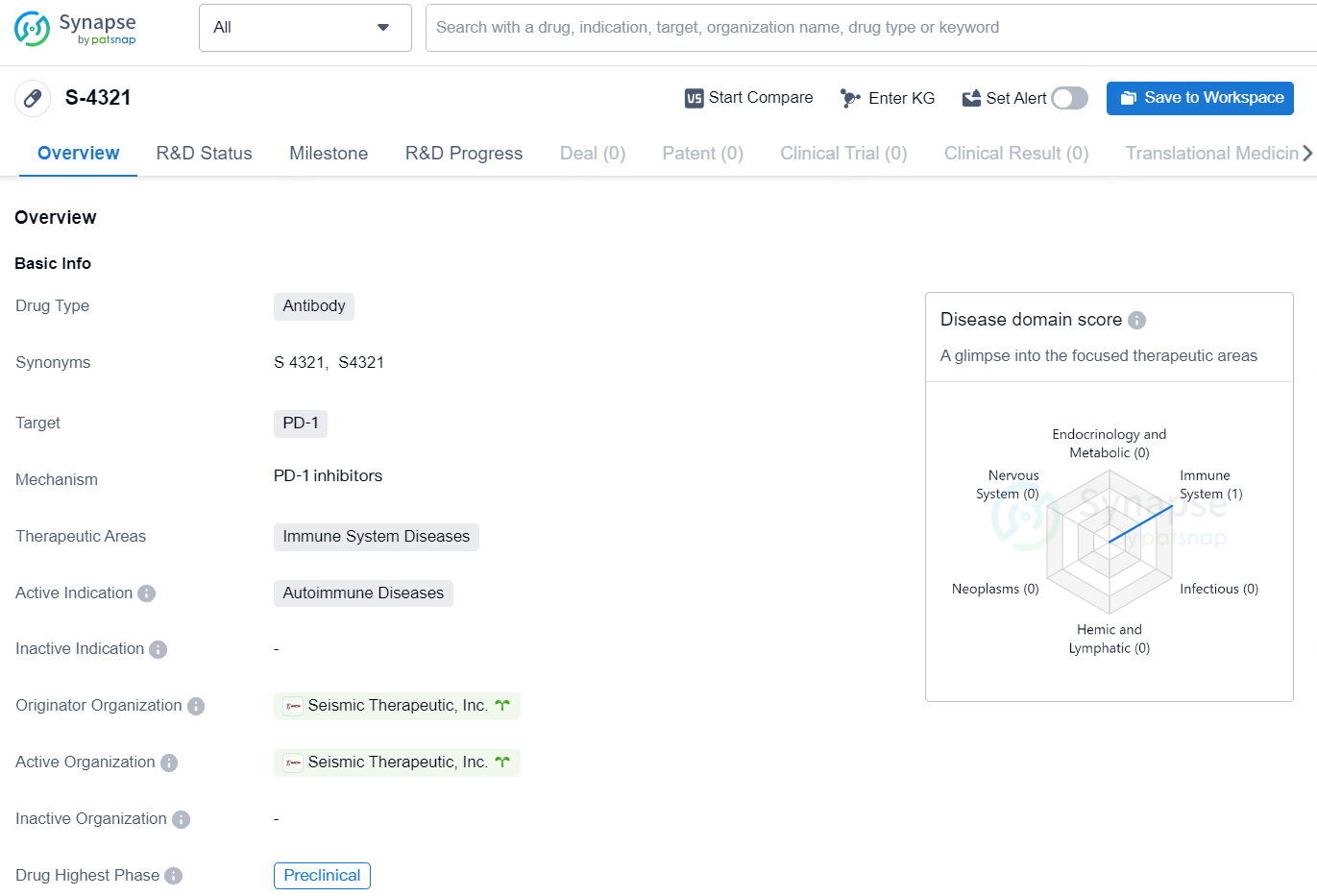
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The presentation at ACR Convergence 2024 highlighted promising preclinical findings that affirm the action mechanism of S-4321 and its development towards clinical application. Utilizing the Company’s IMPACT platform, S-4321 was engineered to activate the inhibitory PD-1 receptor on T cells similarly to its natural ligand, and to selectively bind to and stimulate the inhibitory FcγRIIb receptor on B cells and antigen presenting cells (APCs) without leading to the depletion of PD-1 expressing cells.
Key points from the poster titled “S-4321, a novel dual-cell bidirectional PD-1:FcγRIIb selective agonist antibody for the treatment of autoimmune disease” include:
An optimized Fab antibody domain with reduced PD-1 binding affinity resulted in sustained PD-1 activation while preventing target depletion or IL-2 production, thereby maintaining inhibition on activated T effector cells.
An Fc antibody domain designed through machine learning showed a selective affinity for the inhibition of the FcγRIIb receptor over other FcγRs, minimizing the release of proinflammatory cytokines that could diminish effectiveness and reduce PD-1+ cell populations, including regulatory T cells (Tregs) necessary for maintaining immune balance. Additionally, the unique Fc domain in S-4321 provides combined inhibition of B cells/APCs along with the PD-1 mediated suppression of T effector cells.
S-4321 was observed to enhance Treg induction and inhibit the progression of graft versus host disease (GvHD) in murine models.
Studies in non-human primates exhibited a dose-proportional exposure along with approximately 70% bioavailability of S-4321 following subcutaneous administration, indicating the possibility of a convenient self-administration regimen.
“We intentionally created S-4321 as an innovative bifunctional antibody that effectively agonizes both PD-1 and FcγRIIb inhibitory receptors in a single entity,” stated John Sundy, MD, PhD, Chief Medical Officer and Head of R&D at Seismic Therapeutic. “We anticipate that the unique profile of S-4321 will lead to enhanced drug efficacy and clinical advantages for various common autoimmune disorders. We are eager to initiate clinical trials in healthy subjects by the first half of 2025, where we will evaluate biomarkers related to PD-1 agonist activity that are facilitated by S-4321’s distinct mechanism.”
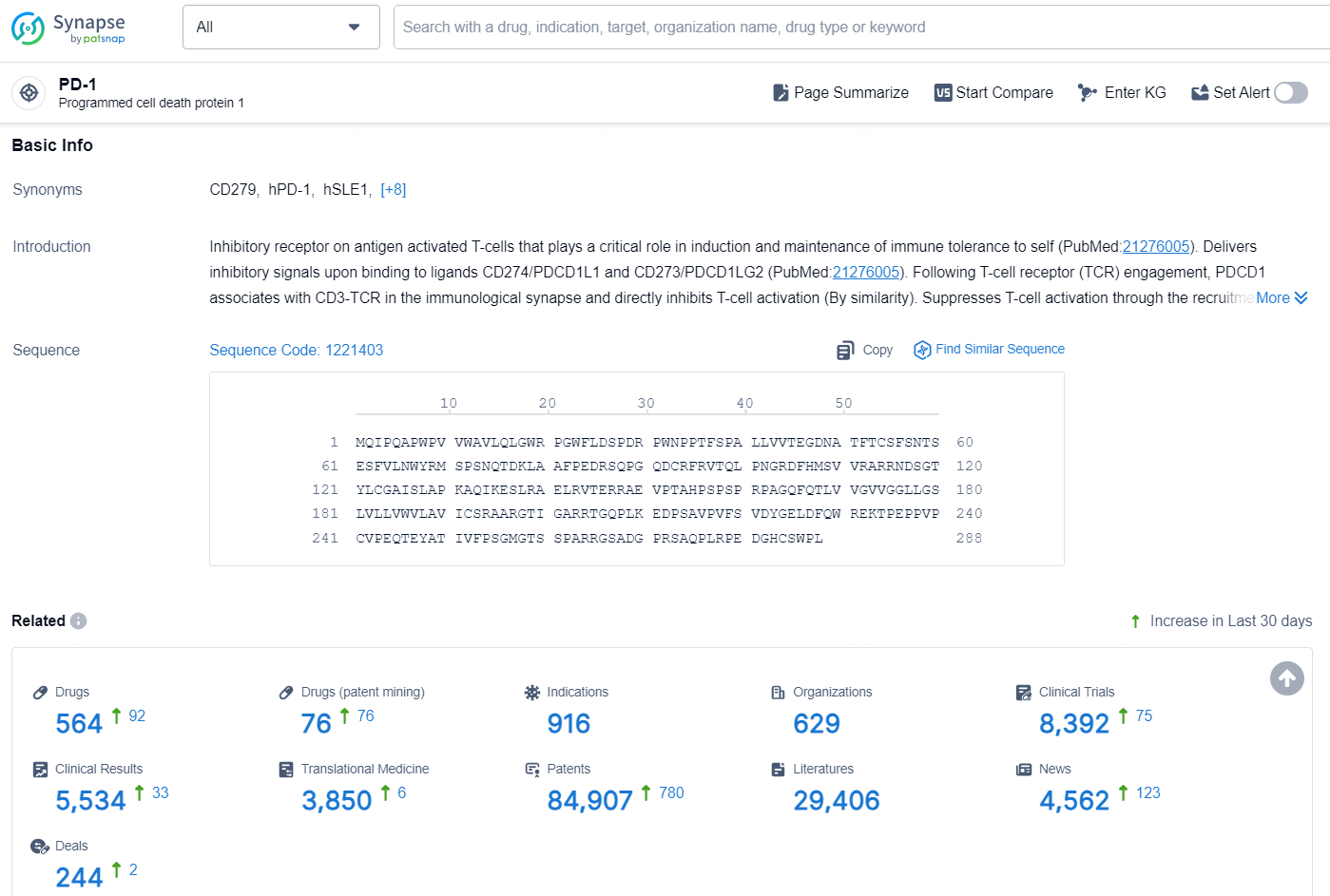
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 564 investigational drugs for the PD-1 target, including 916 indication, 629 R&D institutions involved, with related clinical trial reaching 8392, and as many as 84907 patents.
S-4321 is an antibody drug targeting the PD-1 protein, with a focus on treating autoimmune diseases within the immune system diseases therapeutic area. The drug is currently in the preclinical phase and is being developed by Seismic Therapeutic, Inc.