Advances in Clinical Research on Janus Kinase Inhibitor
Janus kinase inhibitor, often referred to as JAKi, is a type of oral small molecule chemically synthesized drug. This medication targets the four tyrosine kinases of the JAK family, namely JAK1, JAK2, JAK3, and TYK2. Acting as a novel targeted drug for the treatment of autoimmune diseases, JAK inhibitors have already been approved for various indications including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and atopic dermatitis. In addition, several other indications are currently under clinical investigation, including psoriasis, ankylosing spondylitis (AS), ulcerative colitis (UC), and Crohn's disease.
JAK is a family of enzymes that play a crucial role in signal transduction within the human body. These enzymes are involved in the activation of various cell signaling pathways, particularly those related to immune responses and inflammation. JAKs are responsible for transmitting signals from cytokine receptors to the nucleus of cells, thereby regulating gene expression and influencing cellular functions. Dysregulation of JAK activity has been implicated in several diseases, including autoimmune disorders and certain types of cancers. Consequently, pharmaceutical research has focused on developing JAK inhibitors as potential therapeutic agents to modulate these signaling pathways and treat associated conditions.
JANUS KINASE Competitive Landscape
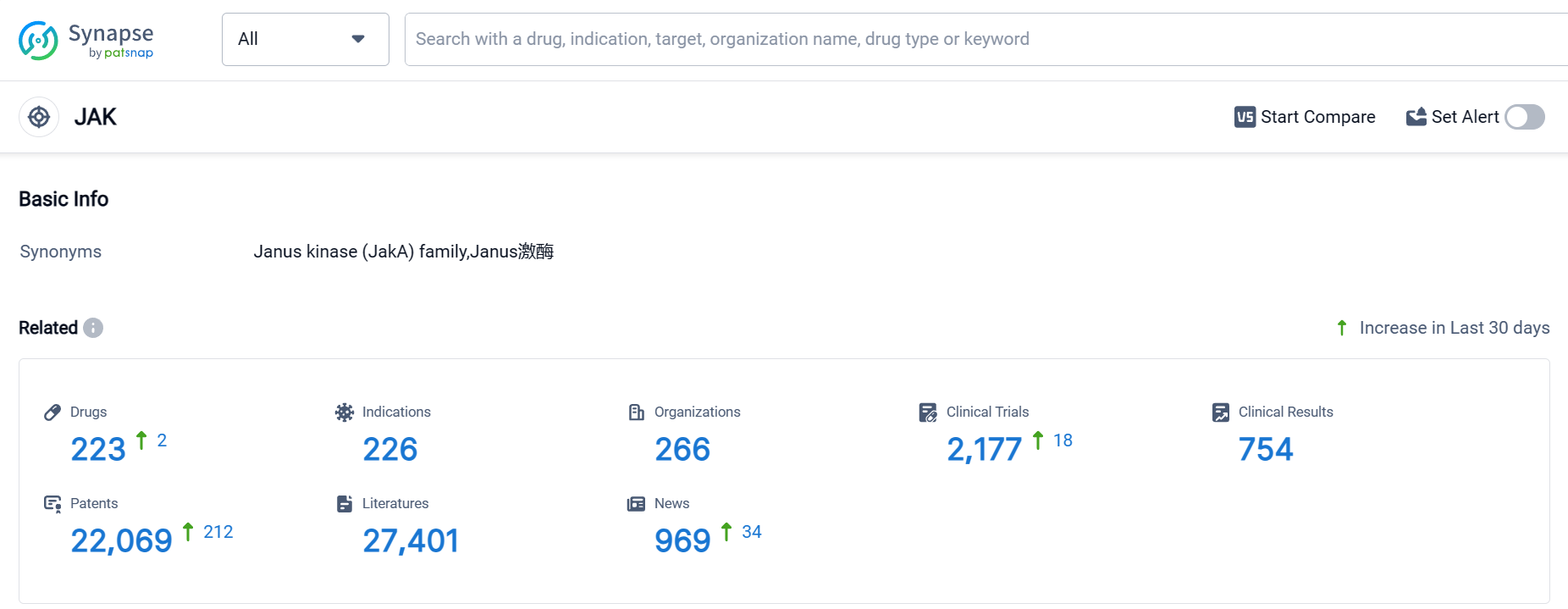
According to Patsnap Synapse, as of 18 Oct 2023, there are a total of 223 JAK drugs worldwide, from 266 organizations, covering 226 indications, and conducting 2177 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis, Pfizer Inc. is the leading company in terms of the highest number of drugs in various phases under the target JAK. The most common indications for JAK drugs include Rheumatoid Arthritis, Dermatitis, Atopic, Colitis, Ulcerative, and Primary Myelofibrosis. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, Japan, European Union, and China are the fastest-developing countries/locations in the target JAK. Overall, the target JAK presents a competitive landscape with significant potential for future development in the pharmaceutical industry.
Key drug: Abrocitinib
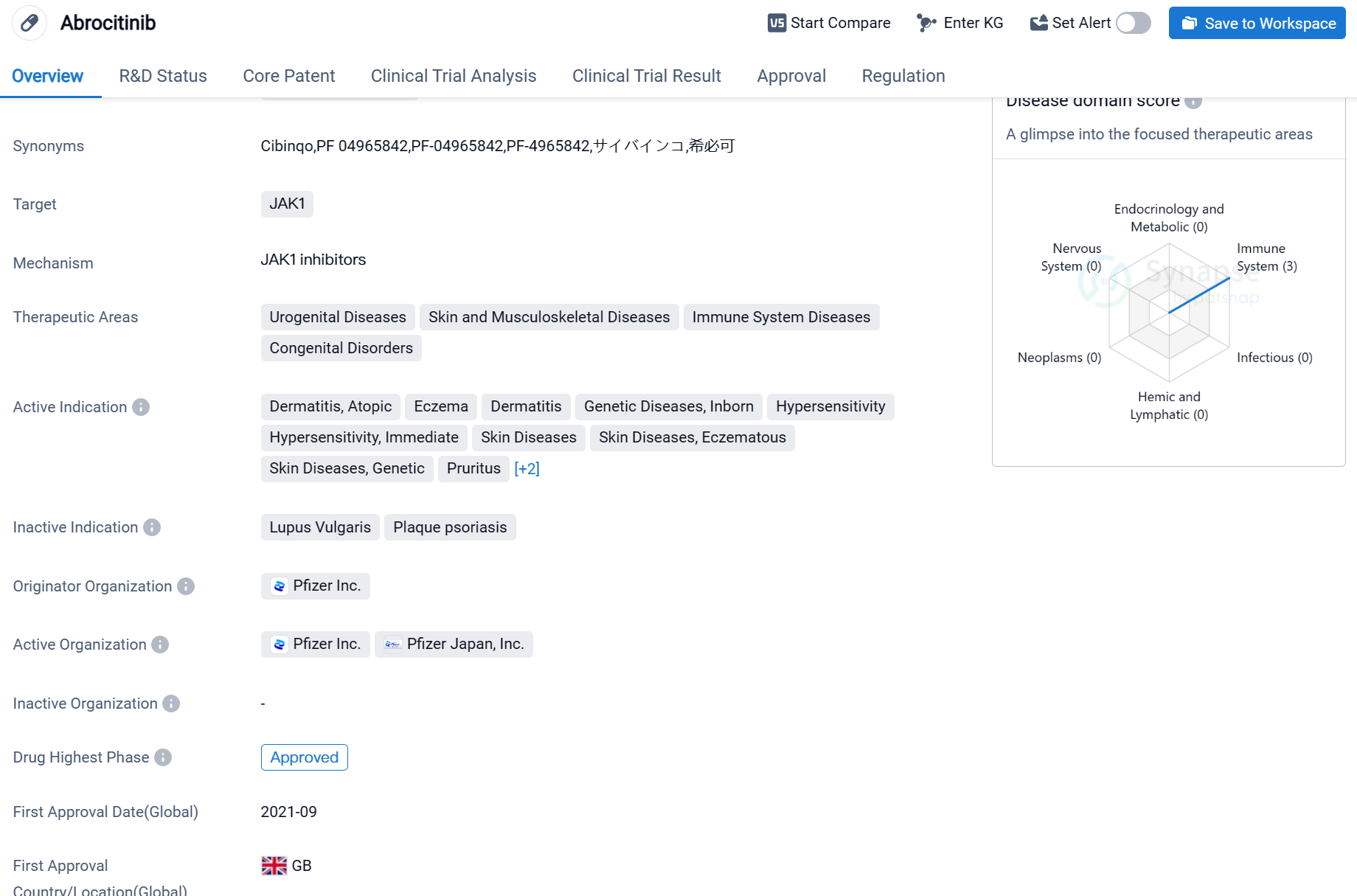
Abrocitinib is a small molecule drug that targets JAK1, a protein involved in various immune responses. It has shown potential in treating a range of therapeutic areas, including urogenital diseases, skin and musculoskeletal diseases, immune system diseases, and congenital disorders. The drug has been specifically indicated for dermatitis, atopic eczema, genetic diseases, inborn dermatitis, hypersensitivity, immediate hypersensitivity, skin diseases, eczematous skin diseases, genetic skin diseases, pruritus, prurigo, and kidney diseases.
Abrocitinib was developed by Pfizer Inc., a renowned pharmaceutical company. It has successfully completed the highest phase of clinical trials and has received approval globally. The drug obtained its first approval in the United Kingdom in September 2021.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of regulatory status, Abrocitinib has been recognized as a Promising Innovative Medicine, indicating its potential to address unmet medical needs. It has also undergone priority review, highlighting its significance in treating the mentioned therapeutic areas. Additionally, Abrocitinib has been designated as a breakthrough therapy, emphasizing its potential to provide substantial improvements over existing treatments.
The objective presentation of this information provides a comprehensive overview of Abrocitinib, a small molecule drug targeting JAK1. Its therapeutic potential spans across various areas, including urogenital diseases, skin and musculoskeletal diseases, immune system diseases, and congenital disorders. The drug has received global and Chinese approvals, with its first approval granted in the United Kingdom in September 2021. Abrocitinib has been recognized as a Promising Innovative Medicine, undergone priority review, and designated as a breakthrough therapy, further highlighting its significance in the pharmaceutical industry.