A Comprehensive Review of sunvozertinib's R&D Innovations and Drug Target Mechanism
Sunvozertinib's R&D Progress
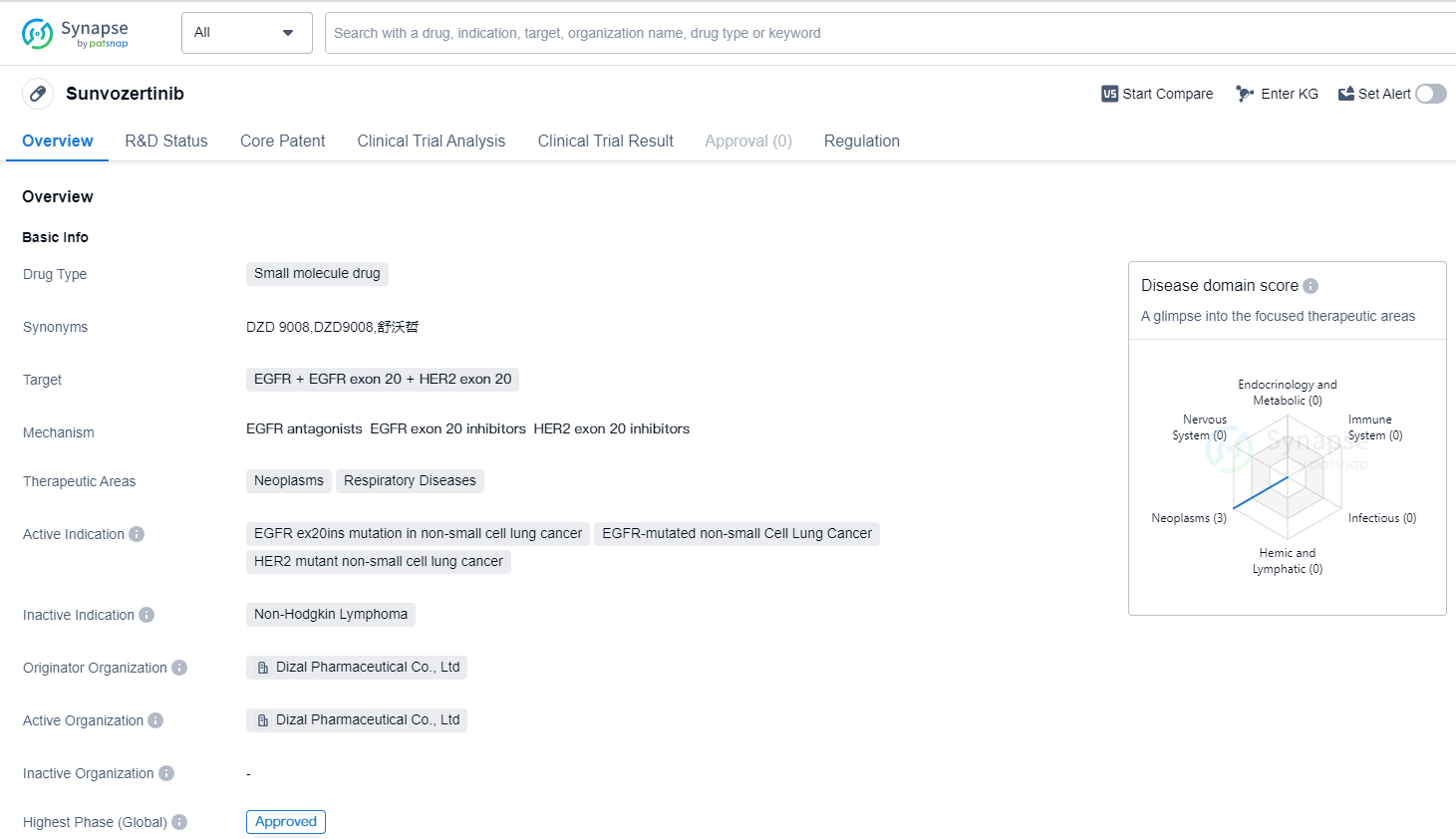
Sunvozertinib is a small molecule drug that falls under the category of biomedicine. It targets EGFR (Epidermal Growth Factor Receptor), EGFR exon 20, and HER2 exon 20. The drug is primarily used in the treatment of neoplasms and respiratory diseases.
Sunvozertinib is specifically indicated for the treatment of EGFR ex20ins mutation in non-small cell lung cancer, EGFR-mutated non-small cell lung cancer, and HER2 mutant non-small cell lung cancer. These indications highlight its potential in addressing specific genetic mutations associated with lung cancer.
The drug was developed by Dizal Pharmaceutical Co., Ltd, an originator organization in the pharmaceutical industry. It has reached the highest phase of development which is approved globally. The first approval for Sunvozertinib was granted in China in August 2023.
In terms of regulatory status, Sunvozertinib has undergone priority review, conditional marketing approval, and has been designated as a breakthrough therapy. These regulatory designations indicate that the drug has shown promising results in clinical trials and may offer significant benefits over existing treatment options.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for sunvozertinib: EGFR antagonists EGFR exon 20 inhibitors HER2 exon 20 inhibitors
EGFR antagonists are drugs that specifically target and inhibit the activity of the epidermal growth factor receptor (EGFR). EGFR is a protein that plays a crucial role in cell growth and division. By blocking the activity of EGFR, these antagonists can help to slow down or stop the growth of cancer cells that rely on EGFR signaling for their proliferation. They are commonly used in the treatment of various types of cancer, such as lung cancer and colorectal cancer.
EGFR exon 20 inhibitors are a specific type of EGFR antagonist that specifically target a specific region of the EGFR gene called exon 20. Mutations in this region of the EGFR gene can lead to the development of resistance to traditional EGFR inhibitors. Therefore, exon 20 inhibitors are designed to overcome this resistance and provide an effective treatment option for patients with EGFR-mutated cancers.
HER2 exon 20 inhibitors, on the other hand, are drugs that target and inhibit the activity of the human epidermal growth factor receptor 2 (HER2) specifically in the exon 20 region. HER2 is another protein involved in cell growth and division, and mutations in the exon 20 region can result in resistance to HER2-targeted therapies. HER2 exon 20 inhibitors are being developed as a potential treatment option for patients with HER2-mutated cancers who have developed resistance to standard HER2-targeted therapies.
In summary, EGFR antagonists, EGFR exon 20 inhibitors, and HER2 exon 20 inhibitors are all types of drugs that target specific proteins involved in cell growth and division. They are used in the treatment of various types of cancers, particularly those with specific mutations that confer resistance to standard therapies.
Drug Target R&D Trends for sunvozertinib
EGFR (Epidermal Growth Factor Receptor) is a protein found on the surface of cells that plays a crucial role in cell growth and division. EGFR exon 20 refers to a specific mutation in the EGFR gene, where a segment of DNA is altered. This mutation is associated with resistance to certain targeted therapies used in the treatment of cancer. Similarly, HER2 exon 20 refers to a mutation in the HER2 gene, which is also involved in cell growth and division. These mutations are particularly relevant in the context of lung cancer, as they can impact the effectiveness of targeted therapies and require alternative treatment approaches.
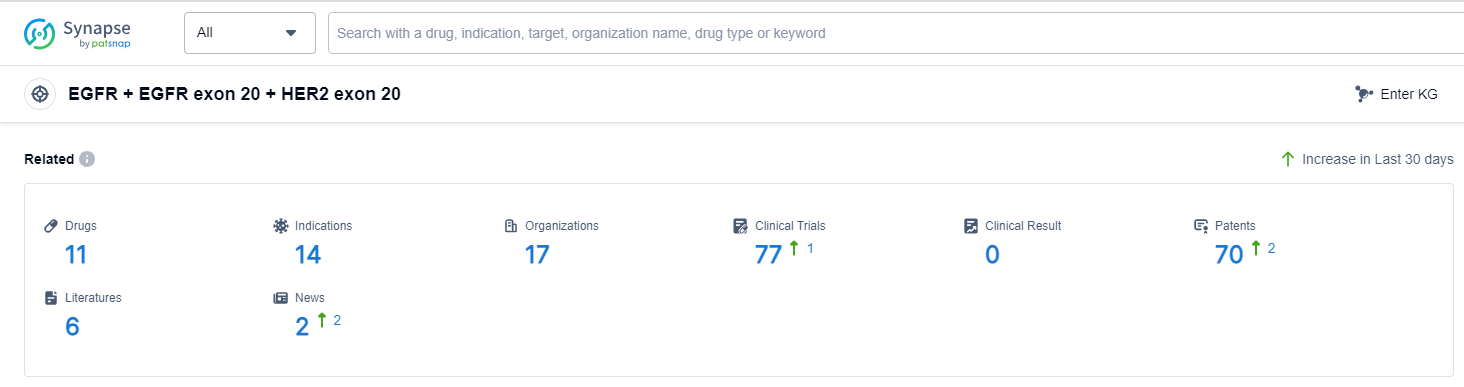
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 11 EGFR + EGFR exon 20 + HER2 exon 20 drugs worldwide, from 17 organizations, covering 14 indications, and conducting 77 clinical trials.
The analysis of the target EGFR + EGFR exon 20 + HER2 exon 20 reveals that Dizal Pharmaceutical Co., Ltd and Takeda Pharmaceutical Co., Ltd. are the companies with the highest stage of development. Drugs under this target have been approved for indications such as EGFR ex20ins mutation in non-small cell lung cancer, Non-Small Cell Lung Cancer, HER2 mutant non-small cell lung cancer, Breast Cancer, Head and Neck Neoplasms, Stomach Cancer, Lung Cancer, Glioma, c-Met positive non-small cell lung cancer, Solid Tumors, Colorectal Cancer, and Non-Hodgkin Lymphoma. Small molecule drugs are progressing most rapidly, and China, along with other countries/locations, is developing drugs under this target. The competitive landscape for target EGFR + EGFR exon 20 + HER2 exon 20 is diverse, with multiple companies and countries involved in the research and development of drugs for various indications. The future development of this target will depend on the success of ongoing clinical trials and the regulatory approvals obtained.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Sunvozertinib represents a significant advancement in the field of biomedicine, particularly in the treatment of lung cancer. Its ability to target specific genetic mutations associated with non-small cell lung cancer, combined with its regulatory designations, suggests that it may offer improved outcomes for patients in need of effective treatment options. The drug's approval in China and its potential for global use further solidify its importance in the pharmaceutical industry.