Advances in the Application of HER2 Inhibitors in Tumors
Human Epidermal Growth Factor Receptor 2 (HER2) is a member of the EGFR kinase family, which also includes HER1, HER3, and HER4. HER2 can form heterodimers closely with other family members, enhancing the activation of the downstream signaling pathway mediated by kinases. HER2 itself lacks known ligands, and HER3 lacks kinase activity. The combination of HER2 and HER3 can form the strongest signaling unit, activate key signaling pathways, including the PI3K-AKT pathway, which is closely related to the occurrence and development of most tumors.
Amplification, over-expression, and mutations of HER2 have been found in many tumors and are widely distributed. For example, the amplification rate is 20% in breast cancer, over-expression rate is 15% - 20%, and mutation rate is 2%; in stomach cancer, the amplification rate is 11% - 16%, the over-expression rate is 20%, and the mutation rate is 3%. HER2 gene changes can be detected in other cancers such as ovarian cancer, uterine cancer, cervical cancer, lung cancer, bile duct carcinoma, pancreatic cancer, colorectal cancer, bladder cancer, prostate cancer, etc. However, it should be noted that according to the current results of gastric cancer research, changes in the HER2 target do not necessarily indicate that this type of tumor can produce relevant treatment reactions to targeted treatment with HER2 inhibitors.
HER2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 521 HER2 drugs worldwide, from 537 organizations, covering 150 indications, and conducting 3344 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.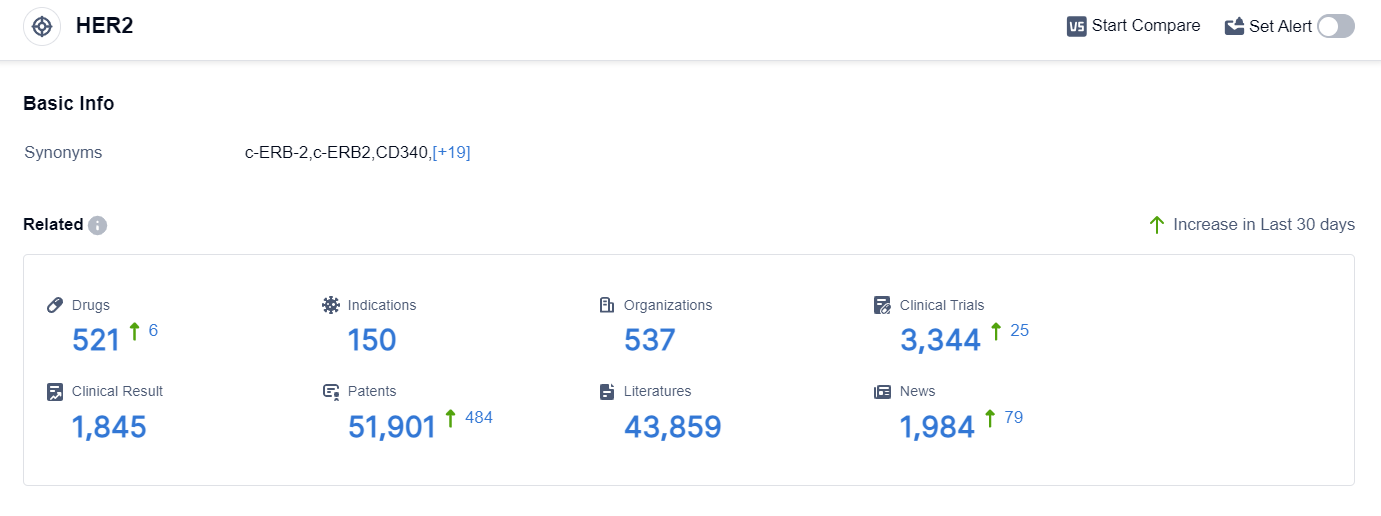
The analysis of target HER2 reveals a competitive landscape with multiple companies actively involved in research and development. Roche Holding AG, Pfizer Inc., and Daiichi Sankyo Co., Ltd. are among the companies with the highest stage of development on this target. The approved indications for drugs targeting HER2 include various types of cancer, such as breast cancer, gastric cancer, lung cancer, and colorectal cancer.
Monoclonal antibodies, biosimilars, and small molecule drugs are the drug types progressing most rapidly under the target HER2. The presence of biosimilars indicates intense competition around the innovative drug, highlighting the importance of intellectual property protection.
The United States, China, and the European Union are the leading countries/locations in the development of drugs targeting HER2. China, in particular, has shown significant progress, with a substantial number of drugs in various stages of development.
Overall, the analysis suggests a competitive landscape in the pharmaceutical industry for target HER2, with ongoing research and development efforts focused on addressing unmet medical needs in various indications. The future development of target HER2 is expected to continue with advancements in therapeutic approaches and potential collaborations between companies in different regions.
Monoclonal Antibody: Margetuximab
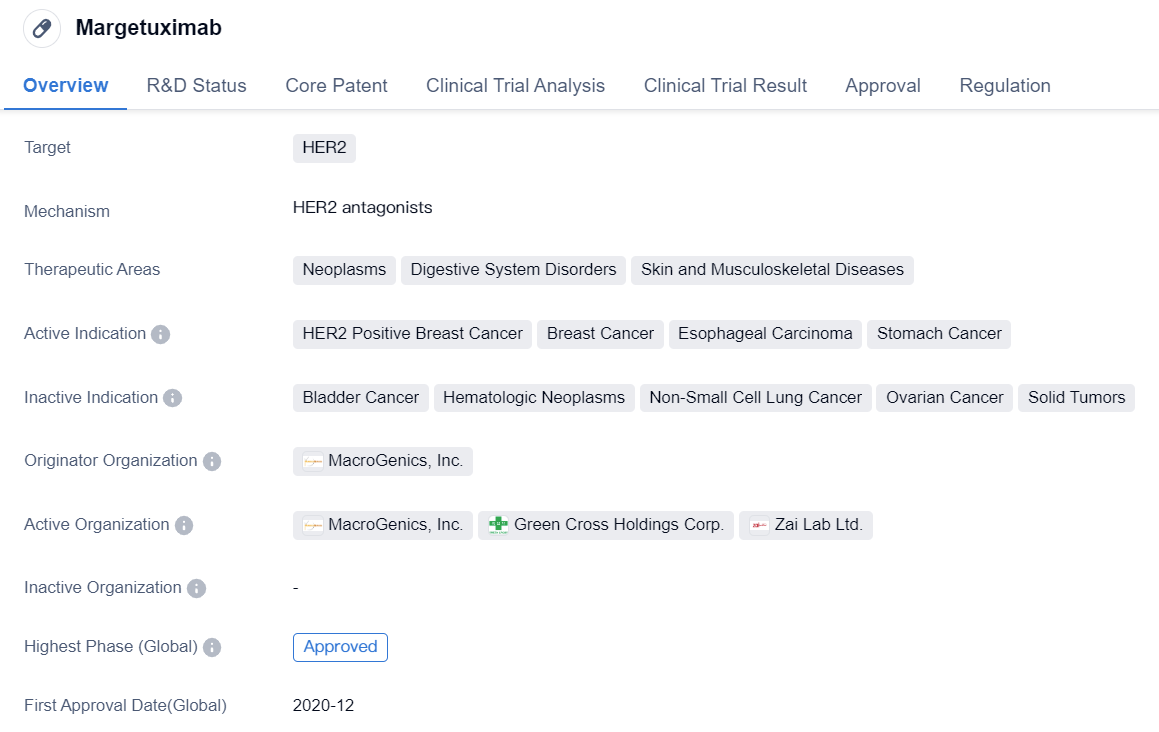
Margetuximab is a monoclonal antibody drug developed by MacroGenics, Inc. It falls under the category of biomedicine and is primarily used for the treatment of various neoplasms, digestive system disorders, and skin and musculoskeletal diseases. The drug specifically targets HER2, a protein that is overexpressed in certain types of cancers.
The active indications for Margetuximab include HER2 positive breast cancer, breast cancer, esophageal carcinoma, and stomach cancer. This indicates that the drug is primarily used in the treatment of these specific types of cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Margetuximab has received approval for use in both the global market and China. Its highest phase of development, which is the phase at which it received approval, is approved. The drug obtained its first approval in December 2020 in the United States. This suggests that it is a relatively new drug in the market.
In terms of regulation, Margetuximab has been granted Fast Track designation and Orphan Drug status. Fast Track designation is given to drugs that have the potential to address unmet medical needs and allows for expedited development and review processes. Orphan Drug status is granted to drugs that are intended to treat rare diseases or conditions.
In summary, Margetuximab is a monoclonal antibody drug developed by MacroGenics, Inc. It targets HER2 and is primarily used for the treatment of HER2 positive breast cancer, breast cancer, esophageal carcinoma, and stomach cancer. The drug has received approval in both the global market and China, with its first approval obtained in the United States in December 2020. It has been granted Fast Track designation and Orphan Drug status, indicating its potential to address unmet medical needs and treat rare diseases or conditions.
Small Molecule Tyrosine Kinase Inhibitor: Mobocertinib Succinate
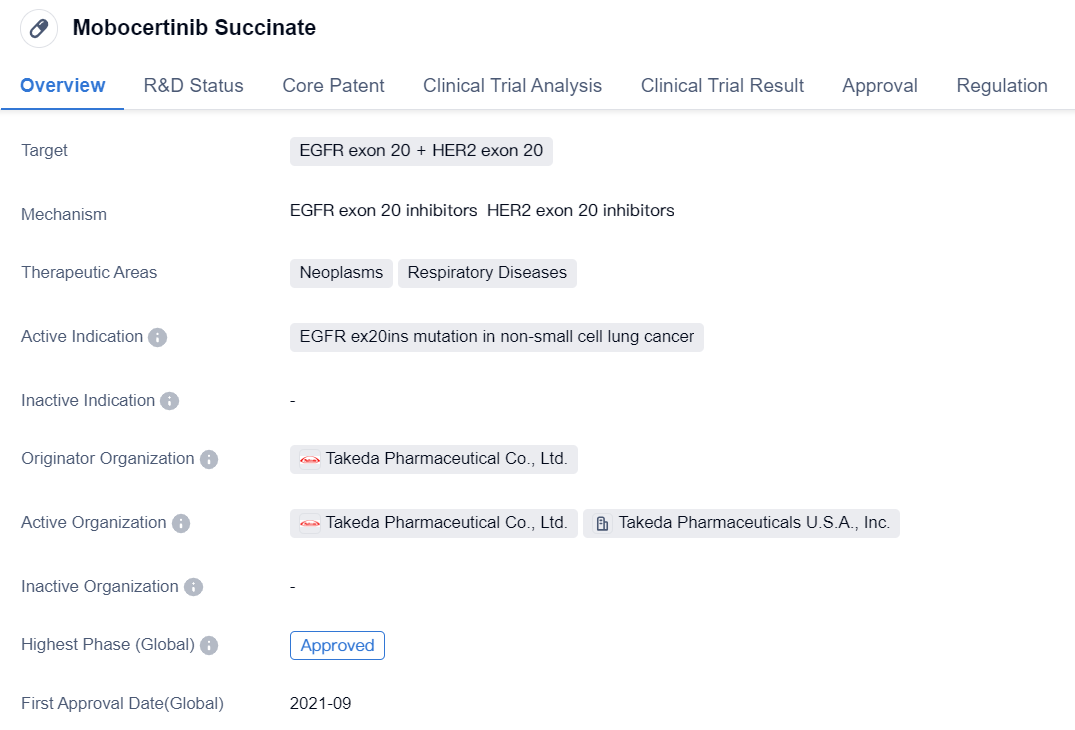
Mobocertinib Succinate is a small molecule drug that falls under the therapeutic areas of neoplasms and respiratory diseases. It specifically targets EGFR exon 20 and HER2 exon 20. The drug is primarily indicated for the treatment of non-small cell lung cancer with EGFR ex20ins mutation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Mobocertinib Succinate is Takeda Pharmaceutical Co., Ltd. It has received approvals for use in both the global market and China. The drug obtained its first approval in the United States in September 2021.
Mobocertinib Succinate has undergone various regulatory processes and has been granted several designations. It has been classified as an orphan drug, indicating its intended use for the treatment of rare diseases. The drug has also received breakthrough therapy designation, which is granted to drugs that show significant improvement over existing therapies for serious conditions. Additionally, it has been granted accelerated approval, conditional marketing approval, fast track designation, and priority review, all of which expedite the regulatory process for drugs that address unmet medical needs.
In summary, Mobocertinib Succinate is a small molecule drug developed by Takeda Pharmaceutical Co., Ltd. It targets EGFR exon 20 and HER2 exon 20 and is indicated for the treatment of non-small cell lung cancer with EGFR ex20ins mutation. The drug has received approvals in the United States and China, and it has been granted various regulatory designations, including orphan drug, breakthrough therapy, accelerated approval, conditional marketing approval, fast track, and priority review.
ADC Drugs: ADO-Trastuzumab Emtansine
ADO-Trastuzumab Emtansine is a drug that falls under the category of monoclonal antibody and antibody drug conjugate (ADC). It is designed to target HER2 and Tubulin, making it suitable for the treatment of neoplasms, skin, and musculoskeletal diseases. The drug has been primarily indicated for breast cancer, specifically HER2 positive breast cancer, metastatic breast cancer, and solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Genentech, Inc. is the originator organization of ADO-Trastuzumab Emtansine. The drug has received approval in both the global and Chinese markets, indicating its effectiveness and safety. The first approval for ADO-Trastuzumab Emtansine was granted in the United States in February 2013.
In terms of regulation, ADO-Trastuzumab Emtansine has undergone priority review, fast track, and breakthrough therapy designations. These regulatory pathways aim to expedite the development and approval process for drugs that address unmet medical needs or demonstrate significant advantages over existing treatments.
ADO-Trastuzumab Emtansine represents a significant advancement in the field of biomedicine, particularly in the treatment of breast cancer. By targeting HER2 and Tubulin, it offers a targeted approach to combating the disease. The drug's approval in both the global and Chinese markets highlights its potential to benefit patients worldwide.