Review and Prospects of HDAC Inhibitors
HDAC (Histone Deacetylase), otherwise known as a histone deacetylase, is an important type of enzyme involved in the process of histone modifications. HDAC can remove the acetyl groups from the lysine residues of histones, altering the charge to create a more compact chromosomal structure, thereby inhibiting gene transcription.
HDAC is an epigenetic regulator, which not only modulates the level of acetylation in histones within chromatin, but also regulates a multitude of proteins associated with cancer progression, cell cycle control, apoptosis, angiogenesis, and cell invasion, among other things. Due to HDAC's fundamental role in gene expression and its varying impacts on histone and non-histone proteins, HDAC has emerged as an ideal target for treating various malignancies. HDAC inhibitors are considered to be one of the most promising anticancer drugs.
HDAC Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 244 HDAC drugs worldwide, from 278 organizations, covering 235 indications, and conducting 1566 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.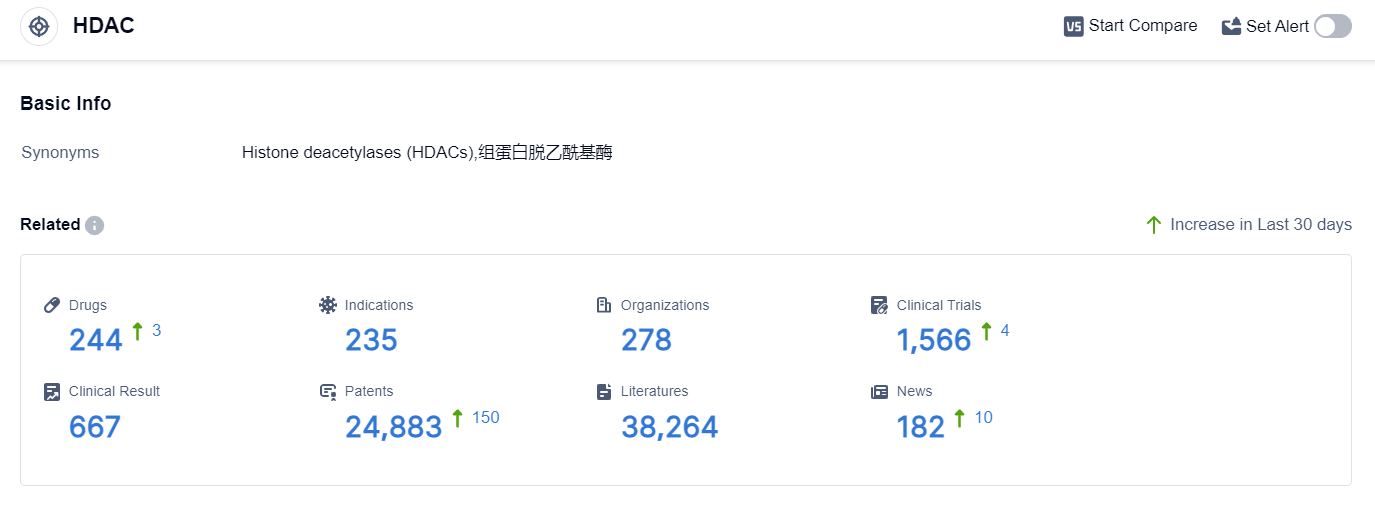
The analysis of the target HDAC from the perspectives of company analysis, indication analysis, drug type analysis, and country/location analysis provides insights into the current competitive landscape and future development of HDAC inhibitors. Bristol Myers Squibb Co., Boao Biology Group Co. Ltd., and Amylyx Pharmaceuticals, Inc. are among the companies growing fastest under the target HDAC.
Several drugs under the target HDAC have been approved for indications such as lymphomas, cancers, neurological disorders, and genetic disorders. Small molecule drugs are progressing most rapidly, indicating intense competition. The development of biosimilars by various research and development institutions further contributes to the competitive landscape.
The United States, Japan, and China are among the countries/locations developing fastest under the target HDAC, with China showing significant progress. Overall, the target HDAC presents opportunities for innovation and growth in the pharmaceutical industry.
Key Drug: Romidepsin
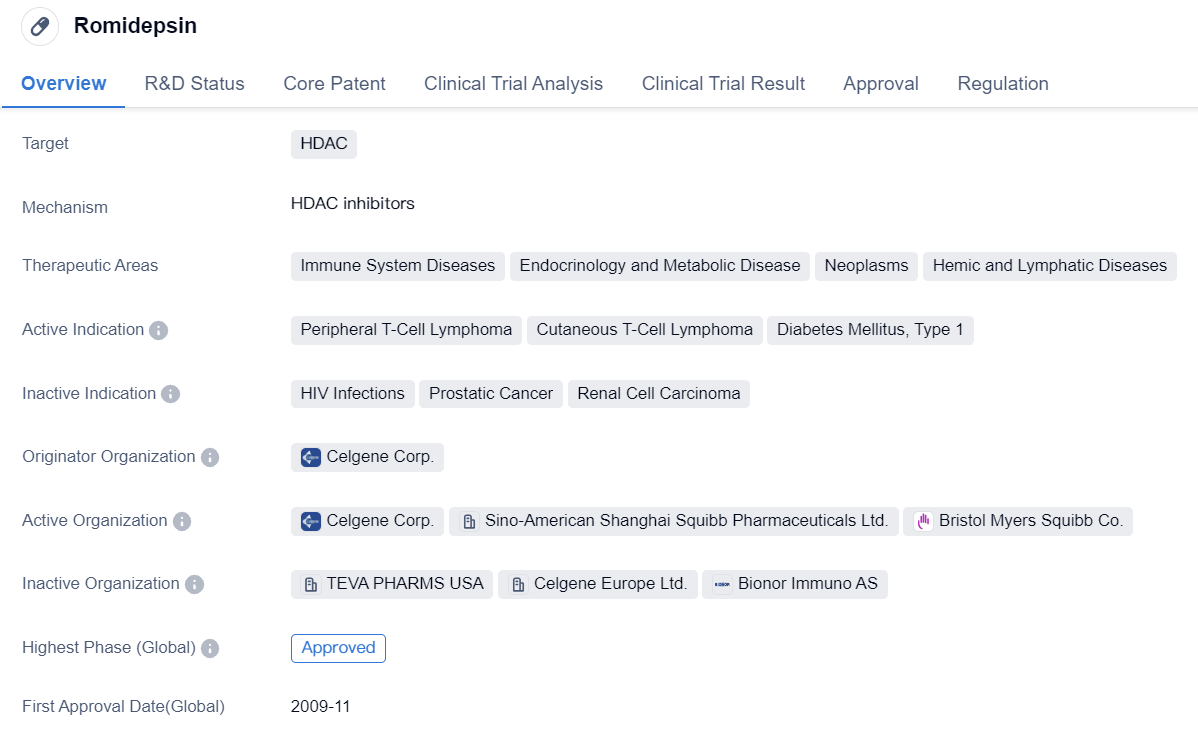
Romidepsin is a small molecule drug that targets HDAC, an enzyme involved in the regulation of gene expression. It has been approved for use in the treatment of various diseases, including peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and type 1 diabetes mellitus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is primarily used in the therapeutic areas of immune system diseases, endocrinology and metabolic disease, neoplasms, and hemic and lymphatic diseases. Romidepsin was first approved for global use in November 2009, with the United States being the first country to grant approval.
Romidepsin was developed by Celgene Corp., a pharmaceutical company specializing in the development of innovative therapies for various diseases. The drug has reached the highest phase of development, which is approval, indicating that it has successfully completed clinical trials and demonstrated its safety and efficacy.
In terms of regulatory status, Romidepsin received accelerated approval, which is a regulatory pathway that allows for the expedited approval of drugs that address unmet medical needs. Additionally, it has been designated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
Overall, Romidepsin is a small molecule drug developed by Celgene Corp. that targets HDAC and has been approved for the treatment of peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and type 1 diabetes mellitus. It has received accelerated approval and orphan drug designation, highlighting its potential to address unmet medical needs in these therapeutic areas.
Chidamide
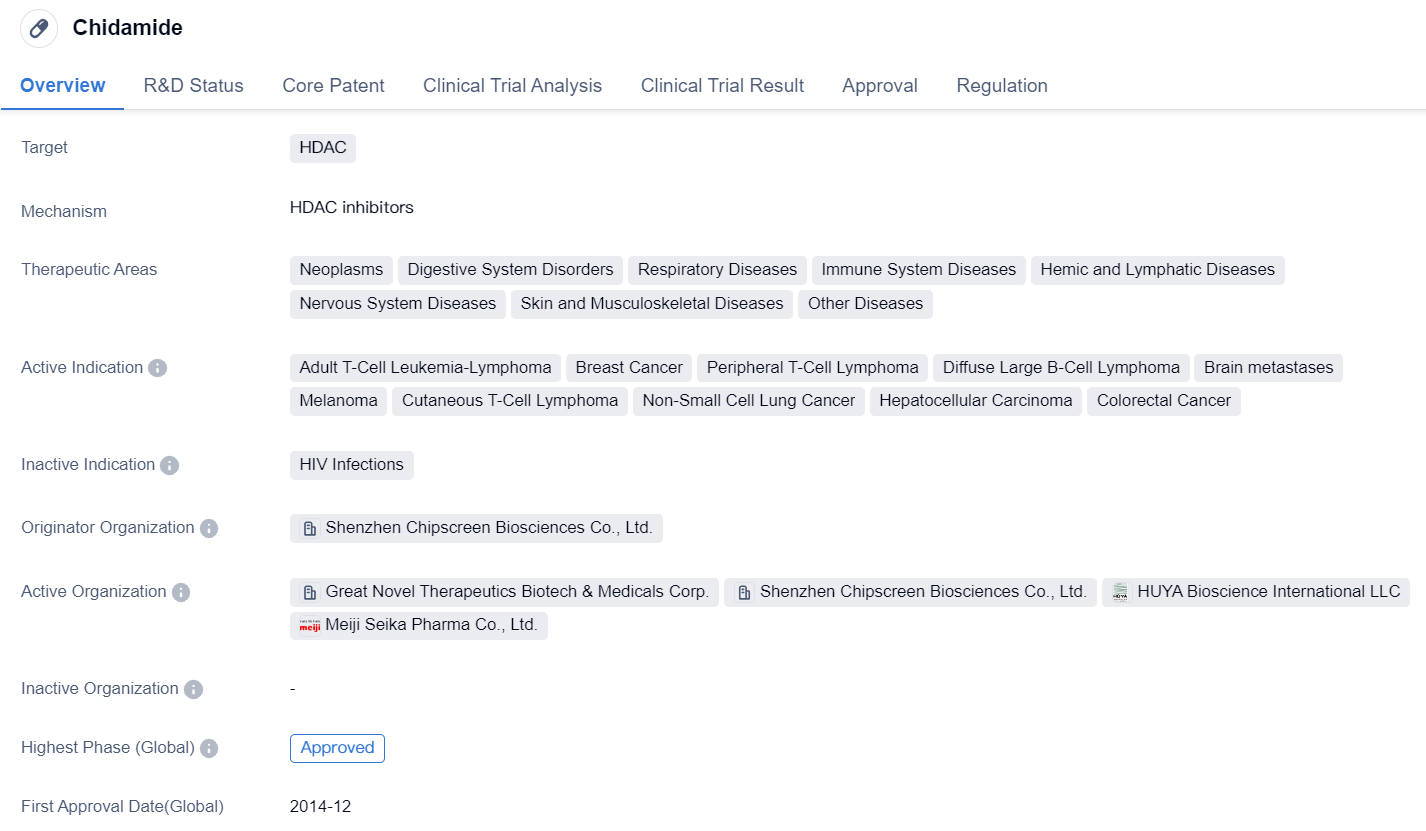
Chidamide is a small molecule drug that targets HDAC, making it a potential treatment option for various diseases. It has been approved for use in several therapeutic areas, including neoplasms, digestive system disorders, respiratory diseases, immune system diseases, hemic and lymphatic diseases, nervous system diseases, skin and musculoskeletal diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating a range of specific indications, such as adult T-cell leukemia-lymphoma, breast cancer, peripheral T-cell lymphoma, diffuse large B-cell lymphoma, brain metastases, melanoma, cutaneous T-cell lymphoma, non-small cell lung cancer, hepatocellular carcinoma, and colorectal cancer. This broad range of indications suggests that Chidamide may have a wide applicability in the field of biomedicine.
Chidamide was developed by Shenzhen Chipscreen Biosciences Co., Ltd., an originator organization based in China. It received its first approval in China in December 2014, and it has also obtained approval in other countries. The drug has reached the highest phase of development, with global and China approvals.
In terms of regulation, Chidamide has been granted priority review status, indicating its potential significance in addressing unmet medical needs. It has also been designated as an orphan drug, suggesting its potential for treating rare diseases. Additionally, Chidamide is part of a special review project, further highlighting its importance in the pharmaceutical industry.
Overall, Chidamide is a small molecule drug that targets HDAC and has been approved for use in various therapeutic areas. Its wide range of indications and the regulatory designations it has received indicate its potential as a treatment option for several diseases. Developed by Shenzhen Chipscreen Biosciences Co., Ltd., Chidamide has achieved global and China approvals, making it a significant player in the field of biomedicine.