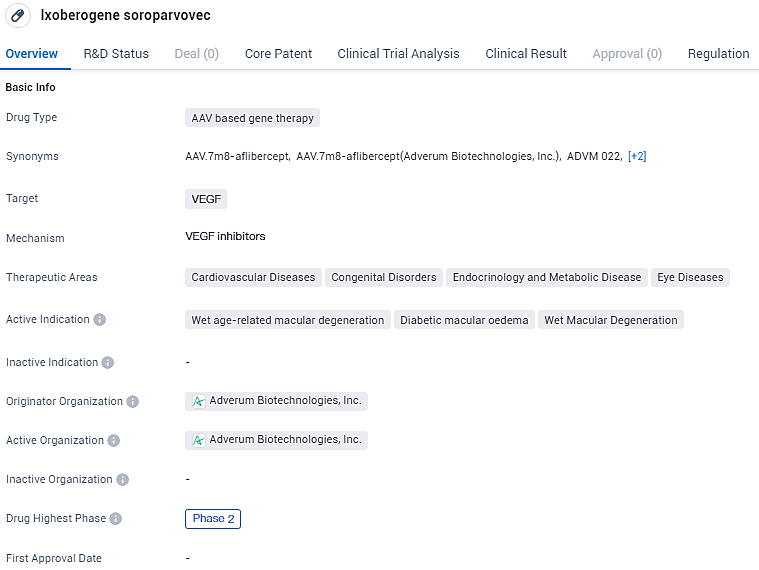
Adverum Biotech's Phase 2 LUNA Study Shows Promising Ixo-vec Results for Wet AMD
Adverum Biotechnologies, Inc., an innovative enterprise in the clinical phase devoted to advancing gene therapy as the novel benchmark treatment for widespread eye conditions, has recently disclosed initial data concerning the safety and efficacy from its current Phase 2 LUNA study, which is examining individuals diagnosed with neovascular age-related macular degeneration.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Wet age-related macular degeneration (wet AMD) stands as a significant cause of vision loss in individuals over 65 years old, often mandating continuous anti-VEGF therapy. "Our aspiration with Ixo-vec is to usher in a new era of nearly injection-free care for those afflicted by wet AMD, with effects that could span multiple years up to a lifetime," declared Laurent Fischer, M.D., the lead executive and CEO of Adverum Biotechnologies.
Expanding on this, Dr. Fischer mentioned, "As we embarked on the LUNA trial, our approach aimed to combine an optimized preventative strategy with a regimen of active doses of Ixo-vec to maximize its enduring efficacy and safety. We are keen to divulge more extensive results from LUNA to pin down the most effective dose and preventative measures for future crucial studies, anticipating the disclosure of our 26-week primary findings by mid-2024."
"Being involved with Ixo-vec for over half a decade in various clinical studies, I'm witnessing remarkable and continuous control of wet AMD symptoms and a notable decrease in treatment frequency for patients post a single Ixo-vec eye injection," commented Arshad M Khanani, M.D., M.A., FASRS, a leading authority and research director at Sierra Eye Associates and an associate clinical professor at the University of Nevada.
"The possibility of Ixo-vec to redefine the approach to wet AMD patient care excites me, and I'm eager to collaborate with Adverum to escalate Ixo-vec through crucial study phases," Khanani added.
Specifically crafted for one-off intravitreal administration within a clinic, Ixo-vec aims to deliver sustained effectiveness, minimize the need for regular anti-VEGF treatments, ensure better adherence from patients, and elevate the overall quality of vision in those suffering from wet AMD. In light of the pressing demand for advanced wet AMD therapies, the FDA has awarded Ixo-vec with the Fast Track designation for its role in addressing this condition.
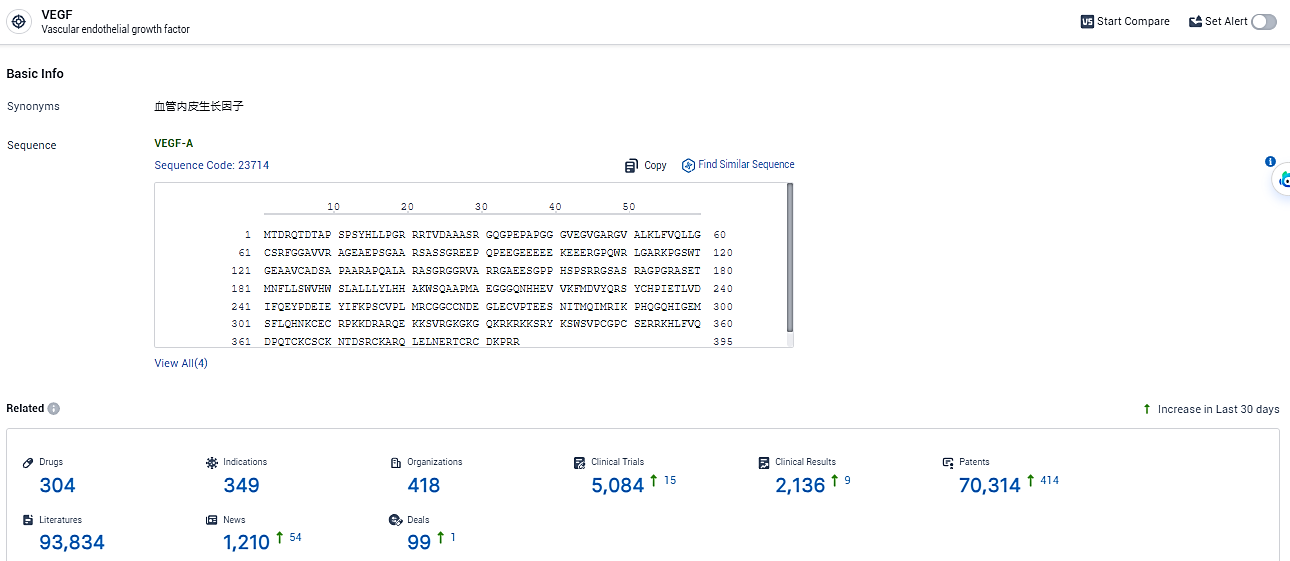
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 304 investigational drugs for the VEGF target, including 349 indications, 418 R&D institutions involved, with related clinical trials reaching 5084, and as many as 70314 patents.
Ixo-vec shows promise as a potential treatment for various cardiovascular, congenital, endocrine, and eye diseases. Its AAV-based gene therapy approach and targeting of VEGF make it a unique and innovative candidate in the field of biomedicine. Further development and regulatory processes will determine its potential for approval and commercialization in the future.