China Grants First-Ever Approval to Crovalimab for Paroxysmal Nocturnal Hemoglobinuria (PNH) Therapy
Chugai Pharmaceutical Co., Ltd. disclosed that the National Medical Products Administration in the People’s Republic of China has sanctioned the use of their innovatively developed humanized complement inhibitor C5 monoclonal antibody, crovalimab. This approval authorizes its application in initiating therapy for adult and adolescent patients diagnosed with PNH who have not undergone any prior treatments involving complement inhibitors.
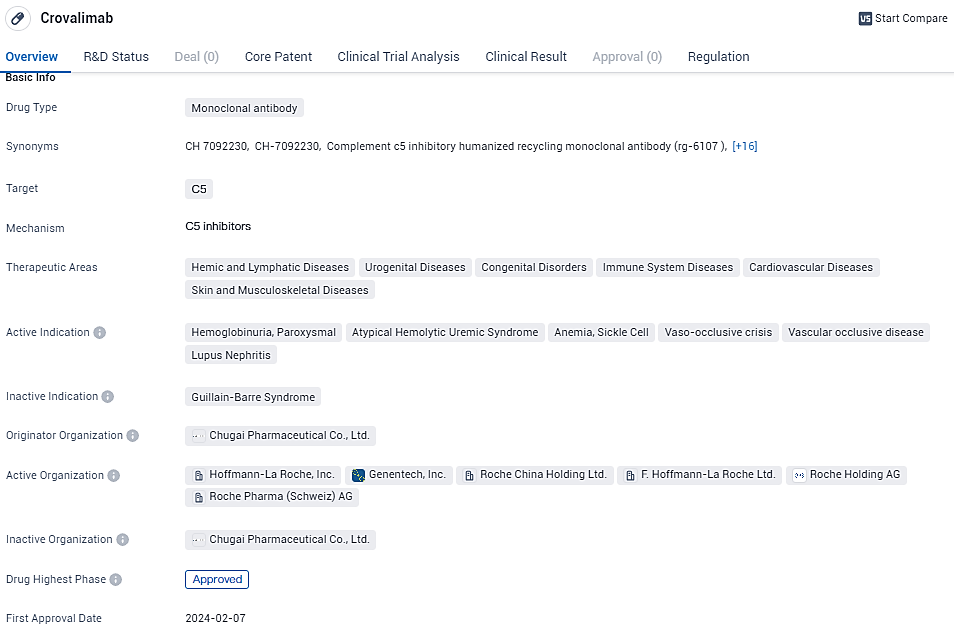
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
With the responsibility for advancing crovalimab's development outside Japan and Taiwan falling to F. Hoffmann-La Roche Ltd., the submission for regulatory consent was undertaken by a Roche subsidiary in China, which has become the inaugural nation globally to sanction the use of crovalimab.
The authorization draws upon data from various research initiatives, notably the COMMODORE 3 study—a phase III, single-arm, multicenter clinical trial executed within China—and the COMMODORE 2 study, which is a global, randomised, phase III, open-label research study assessing PNH patients previously untreated with complement inhibitors.
The innovative crovalimab employs Chugai’s proprietary Recycling Antibody technology. Unlike conventional antibodies, which attach to antigens in a single instance, crovalimab is devised to bind repeatedly to the antigen, fostering persistent inhibition of the complement system through reduced dosage and enabling a subcutaneous dosing regimen every four weeks. Crovalimab is the second therapy sanctioned that utilizes Chugai’s technology, succeeding Enspryng® for neuromyelitis optica spectrum disorder treatment.
Crovalimab, an anti-C5 recycling antibody utilizing Chugai's technology, permits pH-dependent antigen engagement, where a sole antibody molecule can bind numerous times with the antigen, prolonging its active period compared to conventional antibodies. Targeted at C5, a critical element in the complement system, crovalimab is projected to modulate complement activity and is seen as a potentially less burdensome therapy through subcutaneous dosing for patients and caregivers alike. Owing to its unique binding site on complement C5, differing from current antibody medications, crovalimab offers a viable alternative for PNH patients with a particular C5 gene mutation, common in about 3.2% of Japanese PNH sufferers, that inhibits standard antibody medications from binding to C5.
Efforts are underway to gain approval for crovalimab as a novel PNH therapeutic in jurisdictions including Japan, the United States, and the European Union. Additionally, Roche is actively engaging in clinical assessments for the drug's use in treating conditions like atypical hemolytic uremic syndrome (aHUS), as well as spearheading international studies for sickle cell disease (SCD) and lupus nephritis.
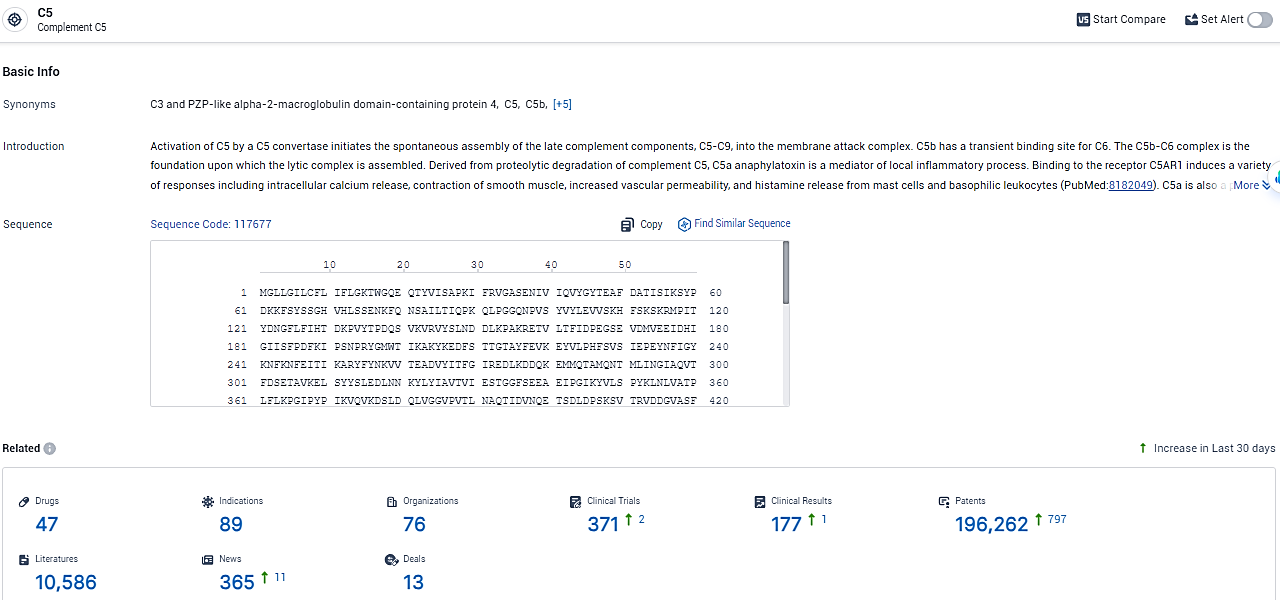
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 19, 2024, there are 47 investigational drugs for the C5 target, including 89 indications, 76 R&D institutions involved, with related clinical trials reaching 371, and as many as 196262 patents.
Crovalimab targets the C5 protein and has been approved for use in China. With its potential to address various diseases and its regulatory designations, Crovalimab represents a significant advancement in the pharmaceutical industry and offers hope for patients suffering from the listed indications.