Almirall Launches Initial Stage Clinical Trial for ALM223, a Modified IL-2 Fusion Protein to Treat Autoimmune Conditions
Almirall S.A., an international enterprise specializing in dermatological healthcare, has recently launched a phase I clinical trial to assess the safety profile, pharmacokinetic properties, immunogenic potential, and pharmacodynamic effects of ALM223. This investigational therapeutic is an altered version of the interleukin 2 protein, specifically an interleukin 2 mutant fusion protein (IL-2 mu-Fc), which is being studied for its potential application across a diverse range of immune-mediated disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
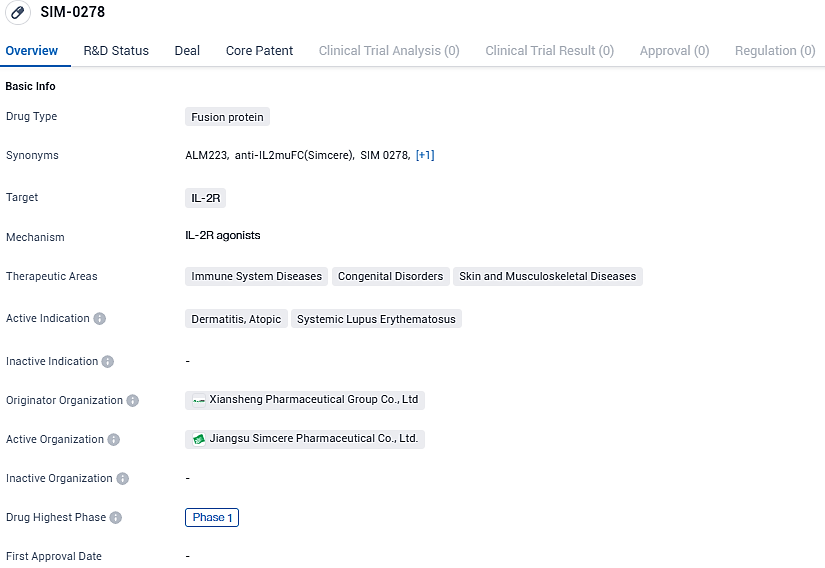
ALM223 represents an enhanced iteration of the IL-2 protein, specifically tailored to target Regulatory T cells for activation. These Tregs play a pivotal role in managing the immune system’s response. By boosting Treg function, it's possible to recalibrate the behavior of additional immune cells, potentially offering a novel therapy for illnesses stemming from an excessively reactive immune apparatus.
Exploratory lab investigations have demonstrated that ALM223 activates Tregs in a focused manner, bypassing any direct impact on different immune cell types like Effector T cells or Natural Killer cells. Consequently, it could be instrumental in reestablishing immunological equilibrium and averting auto-immune reactions where the body's defense system mistakenly assaults its own tissues.
Karl Ziegelbauer, Ph.D., the Chief Scientific Officer at Almirall, expressed enthusiasm about the commencement of human trials for this cutting-edge IL-2 variant, which is also recognized as a significant compound with the capacity to manage a wide range of immune disorders. Dr. Ziegelbauer looks forward to advancements in the clinical trials for this medical product in hopes of enhancing the quality of life for patients affected by immune-related dermatological conditions.
Formerly known as SIM0278, ALM223 was created by Simcere utilizing its sophisticated protein engineering techniques. In a strategic move during September 2022, Almirall secured a deal with Simcere Pharmaceutical, acquiring an exclusive license for IL2-mu-Fc which allows Almirall to spearhead the development and marketing of ALM223 for all indications beyond the Greater China market. Meanwhile, the rights within Greater China are maintained by Simcere Pharmaceutical.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
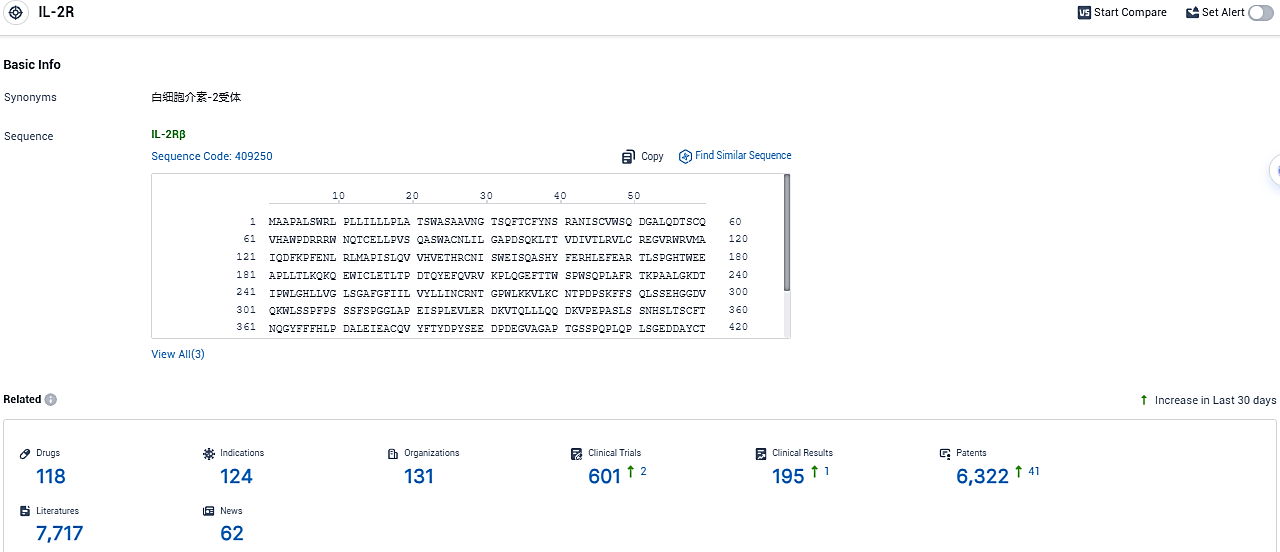
According to the data provided by the Synapse Database, As of December 28, 2023, there are 118 investigational drugs for the IL-2R target, including 124 indications, 131 R&D institutions involved, with related clinical trials reaching 601, and as many as 6322 patents.
ALM223 has potential applications in treating immune system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug is currently in Phase 1 of clinical trials, both globally and in China. Further research and development will be needed to determine the efficacy and safety of ALM223 in treating the indicated conditions.