amgen-announces-phase-iii-study-data-for-kras-g12c-inhibitor-sotorasib
On October 22, 2023, Amgen released the results of their global phase III CodeBreaK 300 study concerning Lumakras (sotorasib, 960mg/240mg) combined with Vectibix (panitumumab, panitumumab monoclonal) in treating chemotherapy-resistant KRAS G12C mutation metastatic colorectal cancer (mCRC). Compared to researchers' chosen drugs (trifluridine+tipiracil, or regorafenib), both dosage groups of sotorasib+panitumumab showed statistically significant advantages in terms of progression-free survival (PFS).
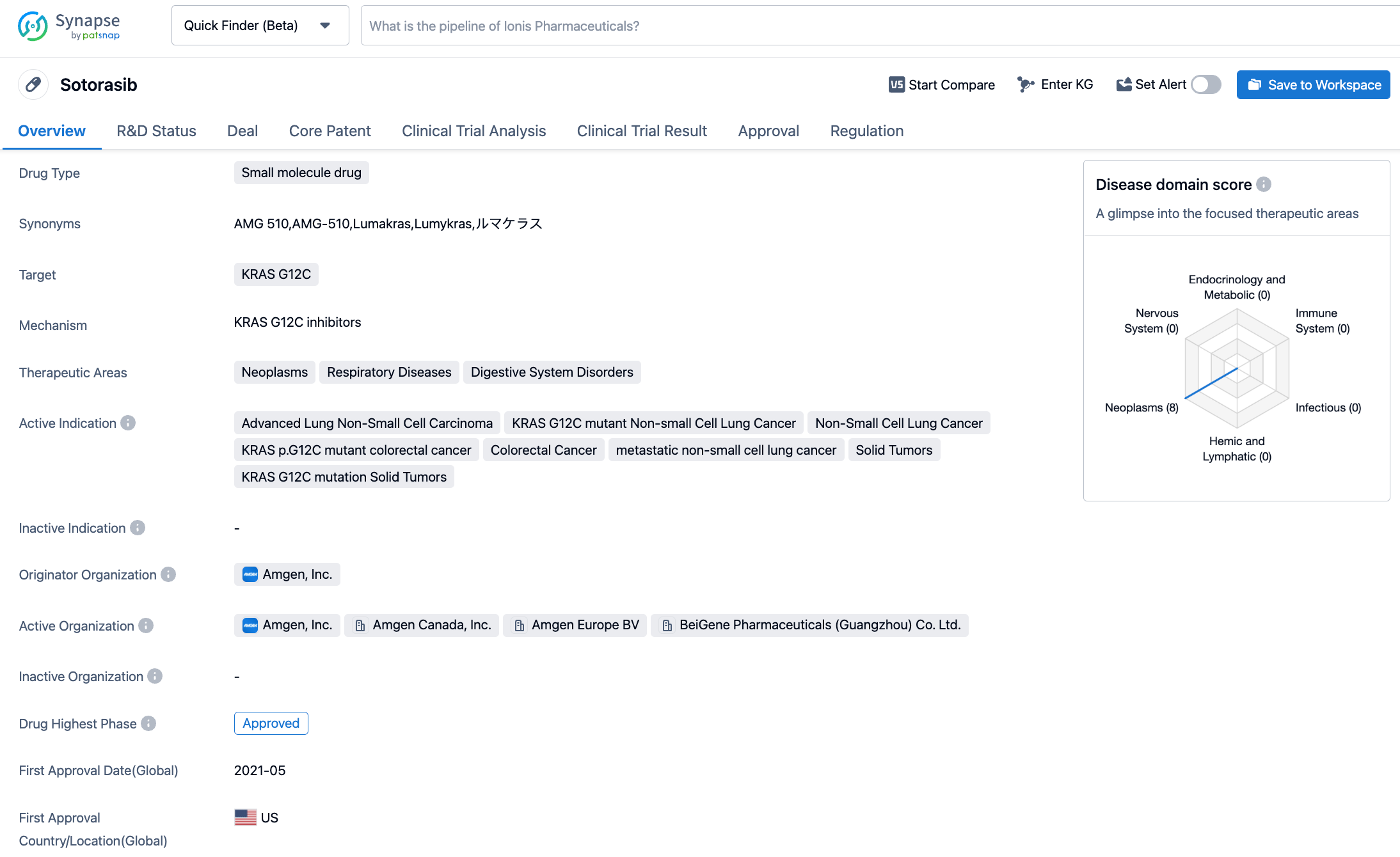
Sotorasib is a KRAC G12C inhibitor medicine jointly developed by Carmot Therapeutics and Amgen, with sales rights in China held by BeiGene. Sotorasib (AMG-510) received accelerated approval from the U.S. FDA in May 2021 for the treatment of adult patients with locally advanced or metastatic KRAS G12C mutation non-small cell lung cancer (NSCLC) who have received at least one previous systemic treatment, as confirmed by an FDA-approved test. This was the world's first anti-cancer drug aimed at KRAS mutations. In September 2022, Amgen announced the results of phase III clinical trial CodeBreaK 200. Compared to standard chemotherapy, Lumakras reduced the risk of disease progression or death by 34% (HR: 0.66, [95% CI: 0.51 to 0.86]; P=0.002). At one year after treatment, the rate of progression-free production in the Lumakras group reached 25%, compared to only 10% in the chemotherapy group. Lumakras also met the secondary endpoint of objective response rate (ORR), with the Lumakras group reaching 28%, compared to 13% in the chemotherapy group (P<0.001). Lumakras also increased the disease control rate (83% compared to 60%). Based on these data, Amgen has filed a supplemental New Drug Application (sNDA) to convert Lumakras' accelerated approval into a full approval, and the FDA is expected to announce its approval results by December 24 of this year.
CodeBreaK 300 is an open-label, global, multi-center, phase III study aiming to evaluate the efficacy and safety of sotorasib combined with panitumumab versus investigator choice of therapy in patients with chemotherapy-resistant KRAS G12C mutation mCRC. A total of 160 participants were randomised in a 1:1:1 ratio to receive treatment with sotorasib (960mg)+panitumumab, sotorasib (240mg)+panitumumab, trifluridine+tipiracil, or regorafenib. The primary endpoint was PFS with secondary endpoints including overall survival (OS) and ORR among others. After a median follow-up of 7.8 months, the median PFS of the sotorasib (960mg)+panitumumab group was 5.6 months (HR: 0.49), while the median PFS of the sotorasib (240mg)+panitumumab group was 3.9 months (HR: 0.58). Patients receiving investigator's choice of therapy had a median PFS of 2.2 months. Another secondary endpoint showed clinically significant improvements in the ORR, disease control rate (DCR), and duration of response (DOR) in both dosage groups. OS has yet to mature. Regarding safety, the primary ≥grade 3 treatment-related adverse events (TRAEs) in the sotorasib+panitumumab treatment group were acneiform dermatitis, hypomagnesemia, rash, and diarrhea. No fatal adverse events were observed.
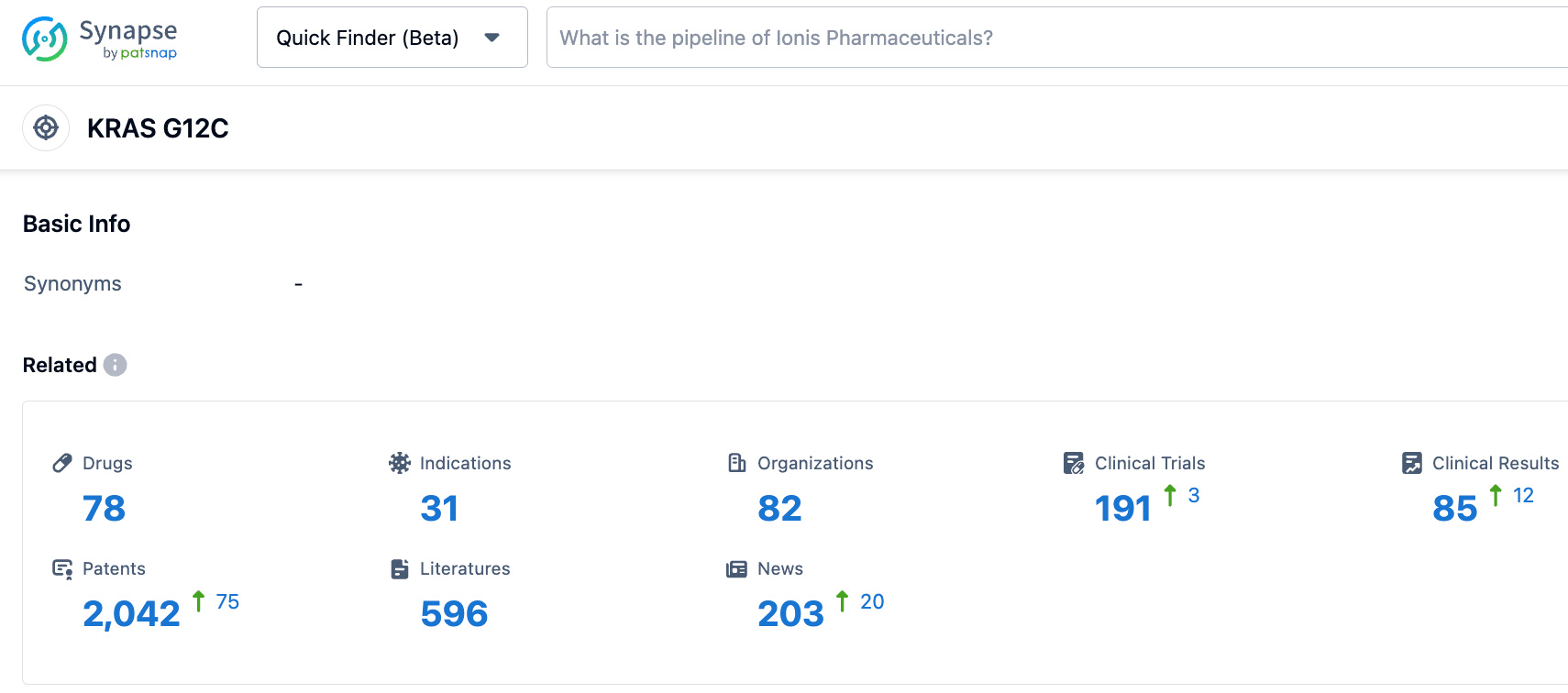
According to information disclosed by the synapse database as of October 24, 2023, there were 78 drugs targeting KRAS G12C under investigation, related indications numbered 31, and 82 institutions were researching them, with 190 clinical trials initiated and as many as 2035 patents applied for. To date, two KRAS G12C inhibitors have been approved by the FDA, and numerous more are currently under clinical development worldwide. The KRAS G12C, once thought of as "undruggable", has now become one of the most closely watched targets in the 'druggable' sphere.