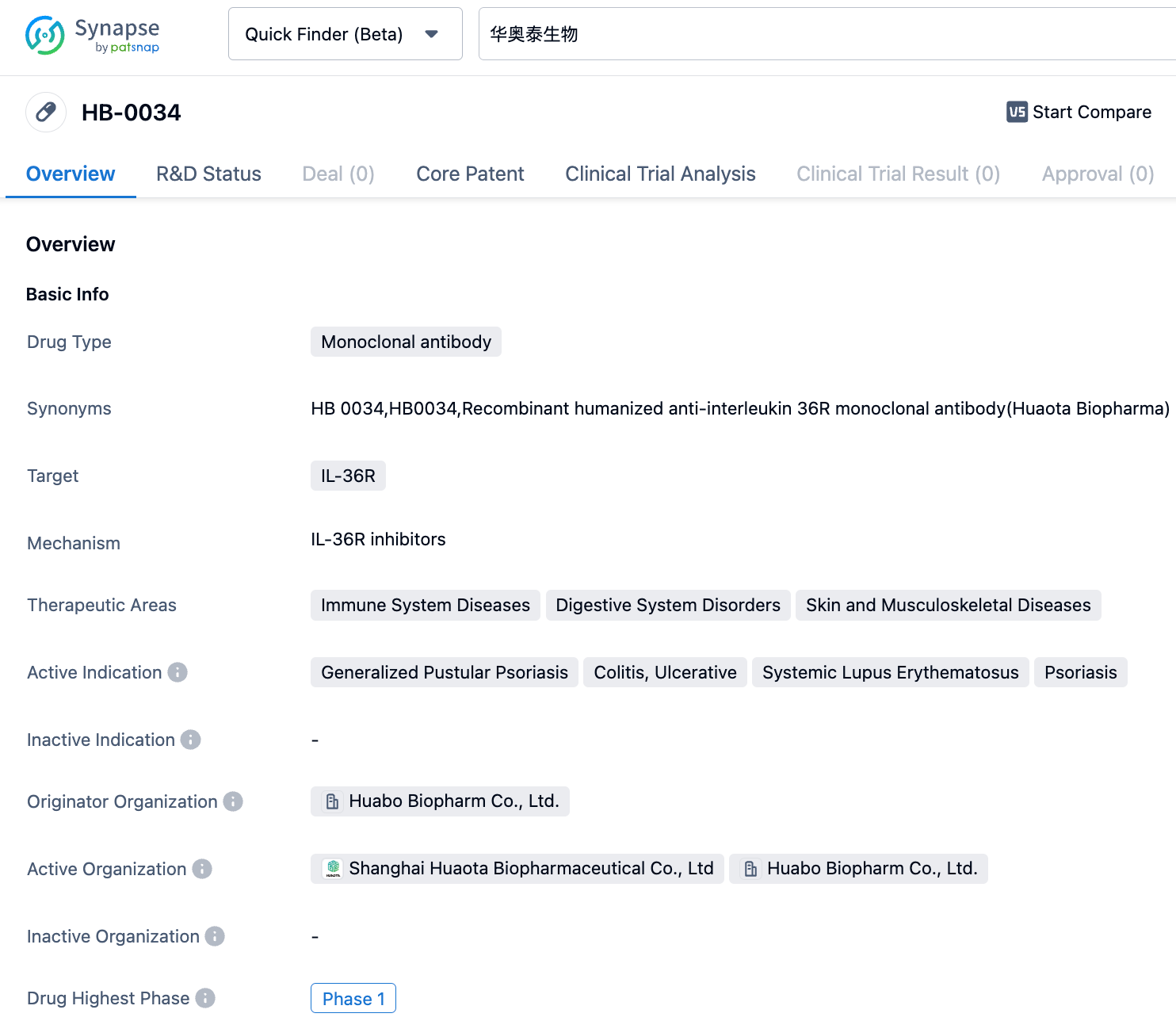
Shanghai Huaota Biopharmaceutical's IL-36R monoclonal antibody, HB0034, has been granted Orphan Drug Designation by the FDA for the treatment of generalized pustular psoriasis
Recently, Shanghai Huaota Biopharmaceutical's HB0034 anti-IL-36R monoclonal antibody injection was granted orphan drug designation by the U.S. FDA for the treatment of generalized pustular psoriasis (GPP).
HB0034 is a humanized IgG1 (Immunoglobulin G1) type monoclonal antibody developed by Huaota Biopharmaceutical targeting IL-36R (Interleukin-36 receptor), which specifically binds to IL-36R, blocking IL-36 inflammatory pathway signals. The monoclonal antibody competitively inhibits the binding of receptor agonists (IL36α, β, and γ) to IL-36R, downregulating downstream pro-inflammatory and pro-fibrotic signal pathways, inhibiting epithelial/fibroblast/immune cell-mediated inflammatory response, reducing the release of inflammatory factors from pathogenic cell in inflammatory/diseases (including GPP, inflammatory bowel disease Systemic lupus erythematosus, fibrotic diseases, etc.) for disease control.
The results of a Phase Ib clinical trial among GPP patients showed that a single dosage of HB0034 could quickly control acute GPP attacks and its effectiveness lasted at least 12 weeks. After a single treatment of HB0034, 77.8% of the patients reached a GPPGA pustule subitem score of 0/1 (no/almost no visible pustules) in the first week, and 44.4% of the patients achieved a GPPGA score of 0/1 (clear or almost clear). Furthermore, the improvement percentage from baseline GPPASI score in the first, fourth and twelfth week after a single HB0034 treatment was 66.4%, 78.7%, and 90.0% respectively. Meanwhile, the improvement percentage from baseline pustular BSA was as high as 92.4% in the first week after a single treatment of HB0034.
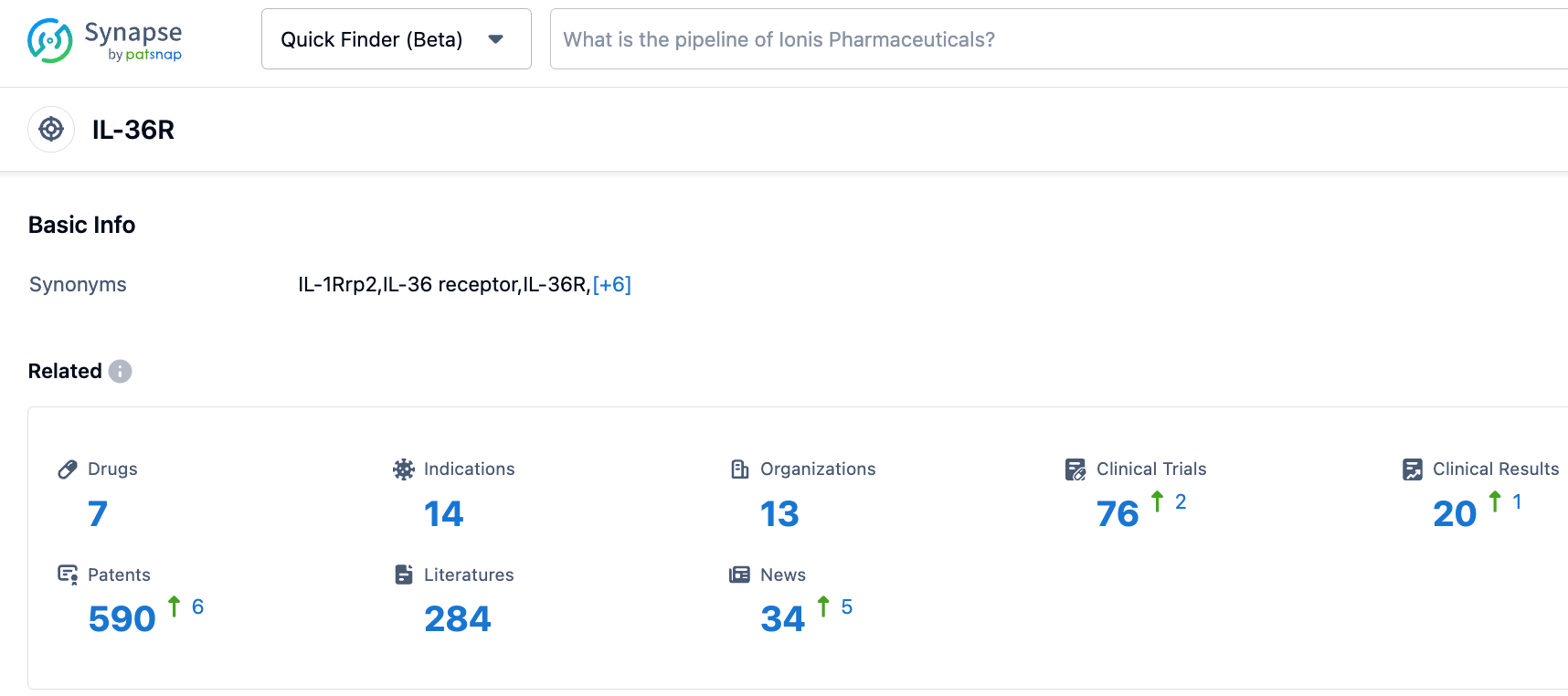
According to information disclosed by the Synapse database, as of October 21, 2023, there are 7 drugs under investigation targeting IL-36R, with 14 indications, among 13 research institutions, with 75 clinical trials and as many as 591 patents. The IL-36R monoclonal antibodies Boehringer Ingelheim's Spesolimab and AnaptysBio's ANB019 (Imsidolimab) have been granted FDA orphan drug designation for the treatment of GPP. HB0034 is the third drug worldwide to receive such recognition. We anticipate successful future developments of HB0034.