Amgen Announces Positive Phase 3 Trial Results for Uplizna® in Treating IgG4-Related Disease
Amgen revealed favorable top-level findings from its Phase 3 clinical trial, which was a randomized, double-blind, multicenter, placebo-controlled study. The trial assessed the efficacy and safety of UPLIZNA® (inebilizumab-cdon) in treating Immunoglobulin G4-related disease (IgG4-RD).
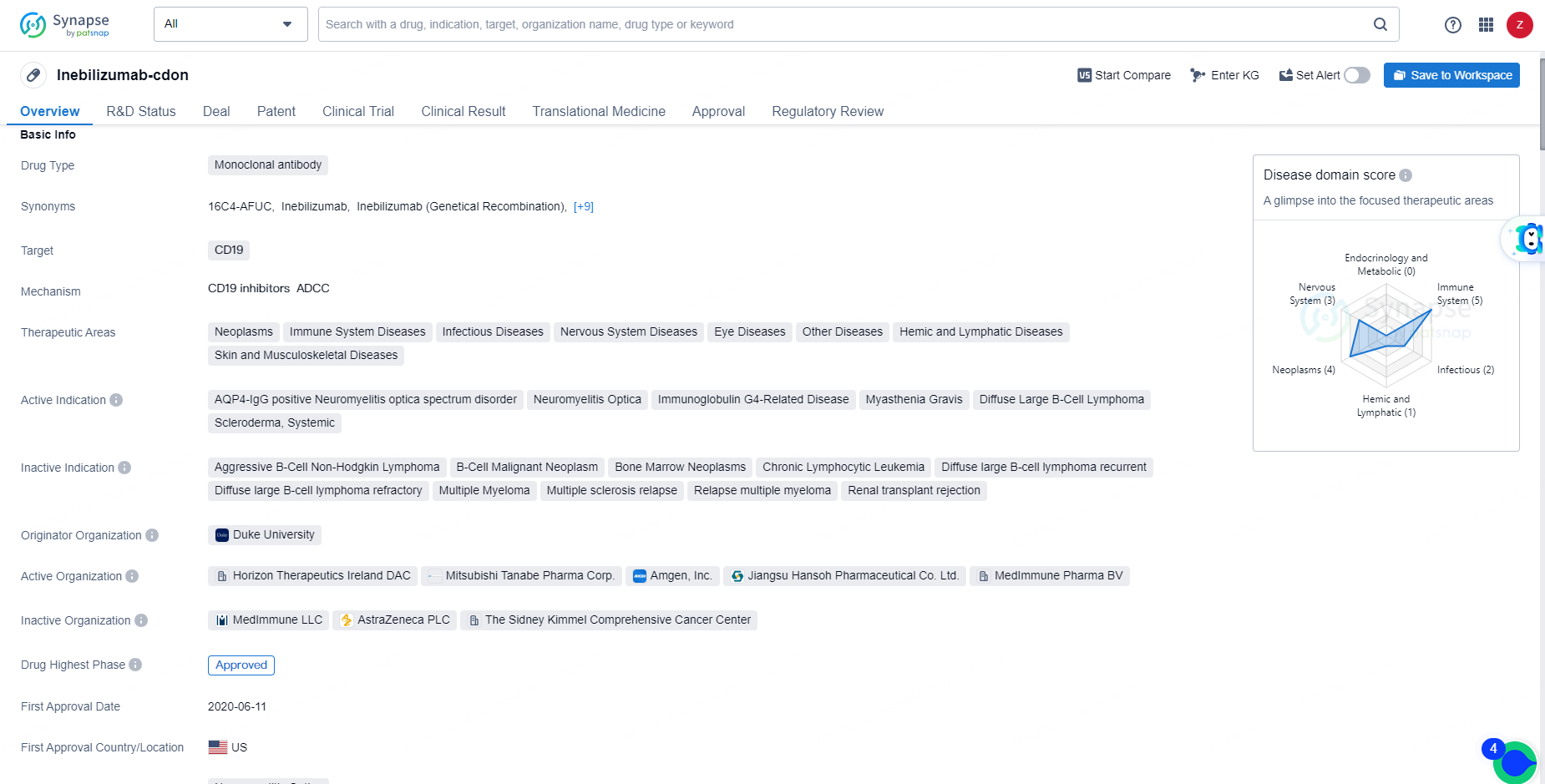
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial achieved its primary objective, demonstrating an 87% statistically significant reduction in the risk of IgG4-RD flare ups compared to a placebo during the 52-week placebo-controlled phase. All important secondary objectives were also fulfilled, including annualized flare rate; flare-free, treatment-free complete remission; and flare-free, corticosteroid-free complete remission. No new safety concerns were identified. The overall safety profile during the placebo-controlled phase was consistent with the established safety profile of UPLIZNA. Complete data from the trial is scheduled to be presented at an upcoming medical conference.
“MITIGATE represents a groundbreaking study with results indicating significant progress in the treatment of patients with IgG4-RD, a rare and devastating condition with no currently approved therapies,” noted Jay Bradner, M.D., executive vice president of Research and Development, and chief scientific officer at Amgen. “We appreciate the collaboration with patients, healthcare providers, and patient advocacy organizations which was crucial for the success of this study. We look forward to making this therapy available to those affected by IgG4-RD.”
The MITIGATE trial was conducted at 80 sites across 22 countries. It is the first placebo-controlled study providing class 1 evidence for the treatment of IgG4-RD, a chronic, systemic, immune-mediated, fibroinflammatory disorder that can affect almost any organ, often involving multiple organs simultaneously, and can lead to irreversible organ damage. The innovative, steroid-sparing study design sets the stage for a treatment approach with reduced toxicity.
“IgG4-RD is a chronic, devastating, immune-mediated disease that has only recently begun to be fully understood,” commented John Stone, M.D., M.P.H., principal investigator and a professor of medicine at Harvard Medical School, also serving as the Edward A. Fox Chair in Medicine at Massachusetts General Hospital. “These findings represent a significant milestone for the IgG4-RD community and offer important insights into how inebilizumab can manage IgG4-RD, as well as crucial understandings about the nature of this disease.”
UPLIZNA is currently approved for Neuromyelitis Optica Spectrum Disorder by several regulatory authorities, including the U.S. Food and Drug Administration, the European Medicines Agency, Health Canada, and the Brazilian Health Regulatory Agency, among others.
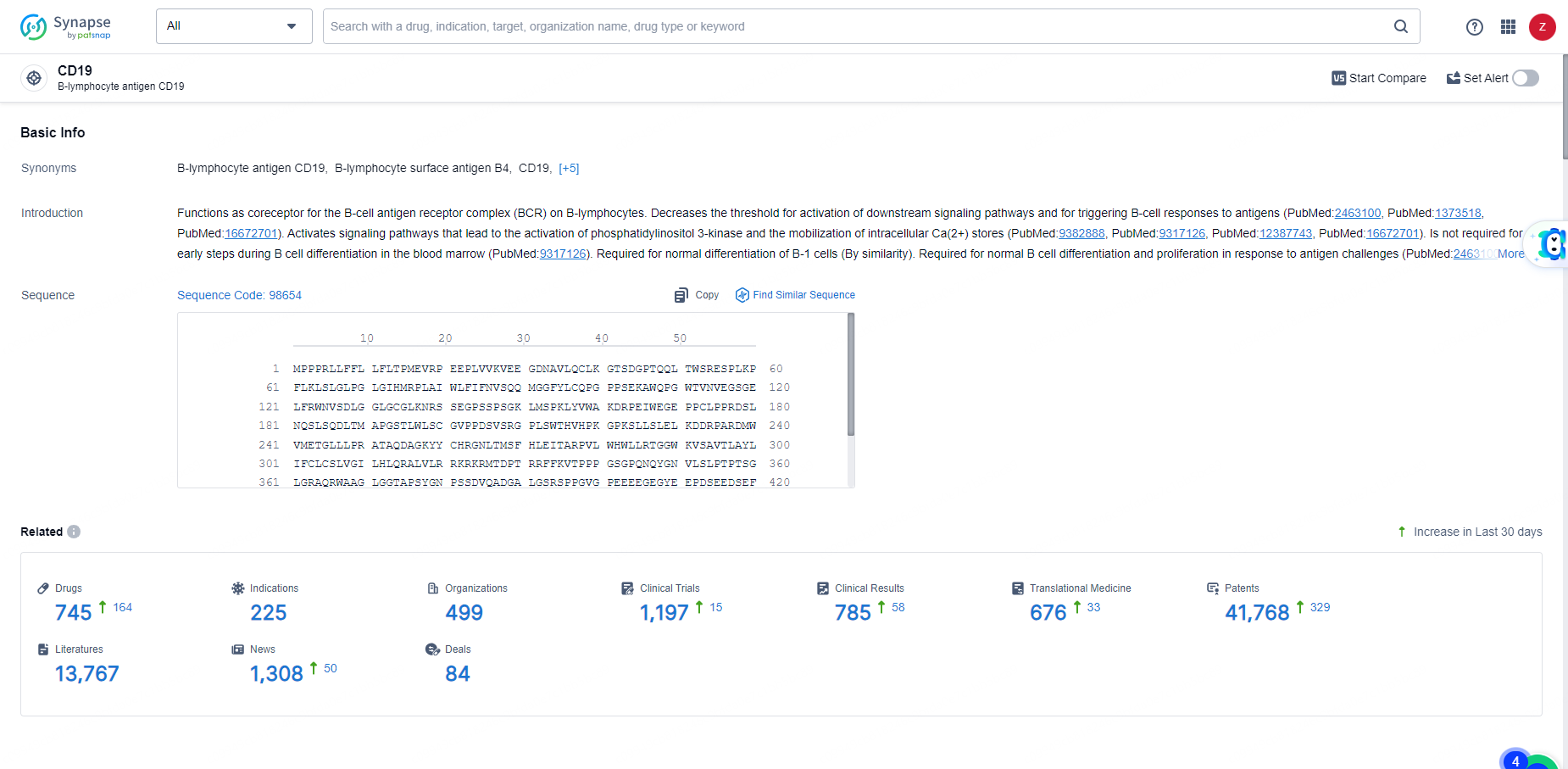
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 11, 2024, there are 745 investigational drugs for the CD19 target, including 225 indications, 499 R&D institutions involved, with related clinical trials reaching 1197, and as many as 41768 patents.
Inebilizumab-cdon is a monoclonal antibody drug that targets CD19 and is used in the treatment of various therapeutic areas including neoplasms, immune system diseases, infectious diseases, nervous system diseases, eye diseases, hemic and lymphatic diseases, and skin and musculoskeletal diseases. Inebilizumab-cdon's approval and regulatory status position it as a valuable addition to the pharmaceutical landscape, offering hope for patients suffering from the specified diseases and demonstrating the continued advancement of biomedicine in addressing complex medical challenges.