An analysis of ADG-126's R&D progress and its clinical results presented at the 2024 ASCO_GI Annual Meeting
On 20 Jan 2024, the latest clinical data of ADG-126 for the treatment of metastatic microsatellite-stable (MSS) colorectal cancer (CRC) were presented in 2024 ASCO_GI. And ADG-126 demonstrated a favorable safety profile and clinical efficacy as monotherapy and in combination with anti-PD-1 therapy.
ADG-126's R&D Progress
ADG-126 is a monoclonal antibody drug developed by Adagene, Inc. It specifically targets CTLA4, a protein involved in regulating the immune system. The drug falls under the therapeutic area of neoplasms, which refers to abnormal growths or tumors. ADG-126 is primarily indicated for the treatment of solid tumors and advanced malignant solid neoplasms.
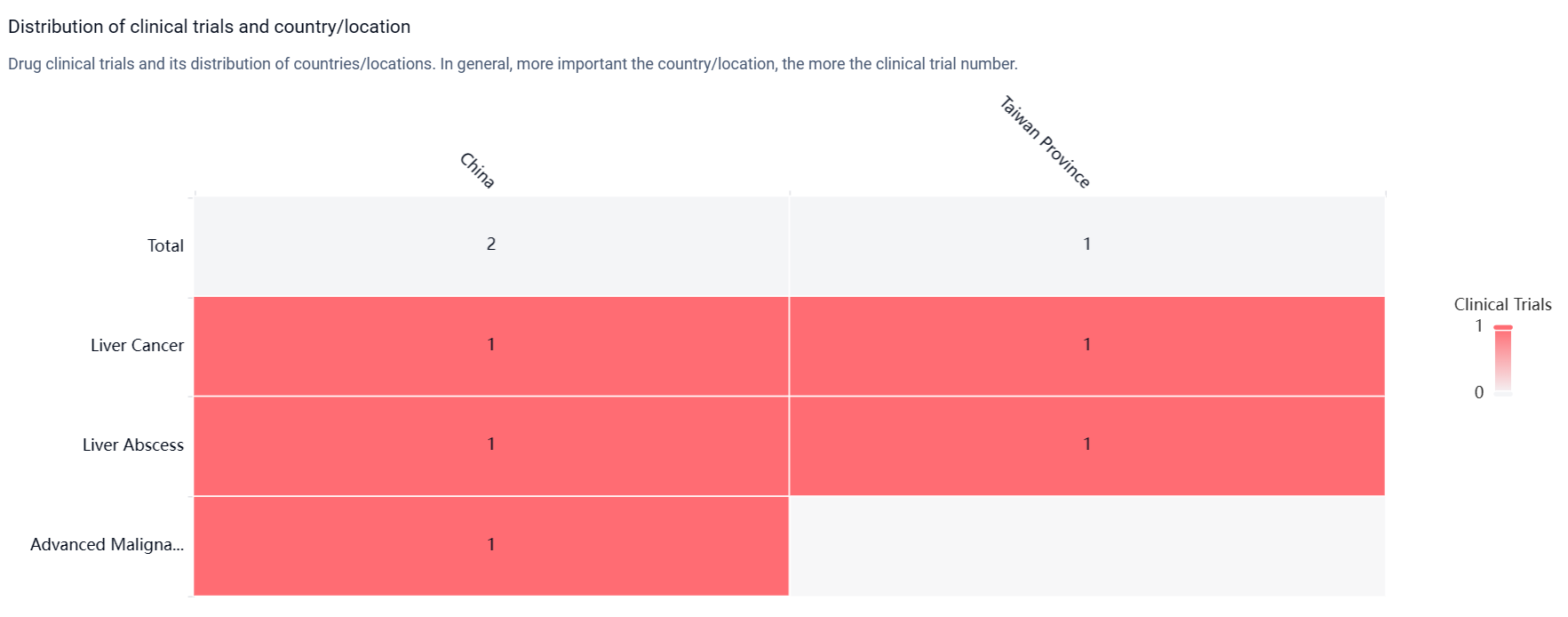
According to the Patsnap Synapse, ADG-126 has reached Phase 2 of clinical development globally. And the clinical trial distributions for ADG-126 are primarily in China and Taiwan Provinnce. The key indication is Liver cancer.
Detailed Clinical Result of ADG-126
This Phase 1b/2, open-label, multicenter dose escalation and expansion study (NCT05405595) was aimed to determine the safety, tolerability and preliminary efficacy of ADG-126 in combo with pembrolizumab (Pembro) in patients (Pts) with metastatic microsatellite-stable (MSS) colorectal cancer (CRC).
In this study, pts received ADG126 [6 or 10 mg/kg (mpk), Q3W or Q6W, IV] plus Pembro (200 mg, Q3W, IV). Primary endpoints are safety and tolerability. Secondary endpoints are PK, ADA, ORR, DCR, DOR and PFS per RECIST 1.1.
The result showed that as of Aug 29, 2023, a total of 47 Pts have been treated in dose escalation (N=11) and expansion cohorts (N=36). The median age is 60.5 yrs (26-78); 31.9% Pts had at least 3 prior therapies, 12.8% Pts received prior IO therapies. A majority of Pts (74.5%) have what are considered immunologically “cold” tumors. ADG126 was dosed at 6 mpk Q6W (1 Pt) and Q3W (5 Pts), 10 mpk Q6W (17 Pts) and Q3W (24 Pts), and Pembro was dosed at 200 mg Q3W. No DLT or Grade 4/5 TRAE was observed at any dose level. TRAEs more than 10% are pruritis (N=9, 19.1%) and diarrhea (N=5, 10.6%). Grade 3 TRAEs were observed in 5 subjects: 1 G3 diarrhea (6 mpk, 16.7%), 1 G3 adrenal insufficiency (10 mpk Q6W, 5.9%), 1 each G3 pancreatitis, blurred vision and lipase increase (10 mpk Q3W, 12.5%); all TRAEs occurred after repeat dosing. For the MSS CRC expansion cohort, 7 SDs at ADG126 10 mpk Q6W dose level (N=11) were observed, and 2 PRs (including 1 initial PR) and 4 SDs at 10 mpk Q3W (N=13) were observed.
It can be concluded that ADG126/Pembro combination is well-tolerated up to the maximally administered dose of 10 mpk Q3W, with limited G3 TRAEs comparable to Pembro monotherapy. The 10 mpk ADG126 Q3W repeat dosing regimen maintains a favorable safety profile and demonstrates anti-tumor activity. The MSS CRC cohort of 10 mpk ADG126 Q3W surpassed the threshold set for part 1 of the Simon’s 2-stage design, and the second stage of the cohort is now enrolling. In conclusion, ADG126/Pembro has a significantly improved therapeutic index over the same class agents in combination with anti-PD-1 therapy, and encouraging early outcomes in patients of MSS CRC without liver metastasis.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
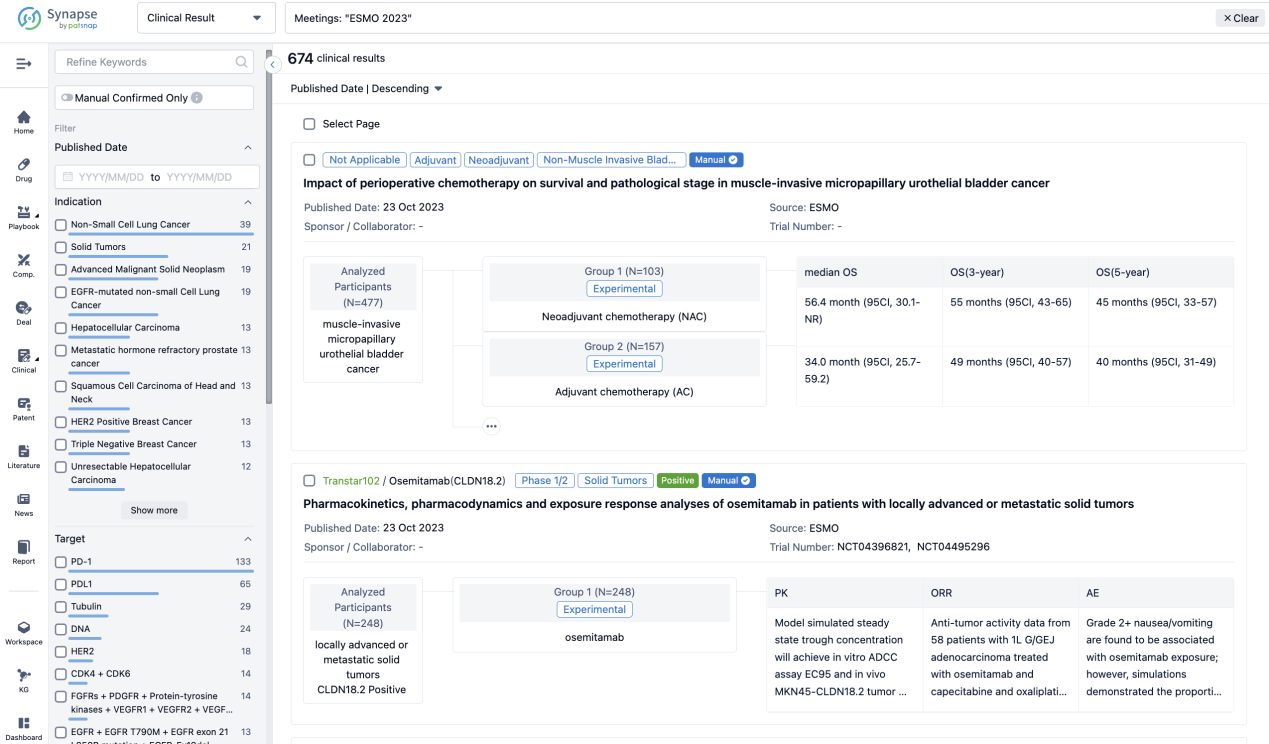
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
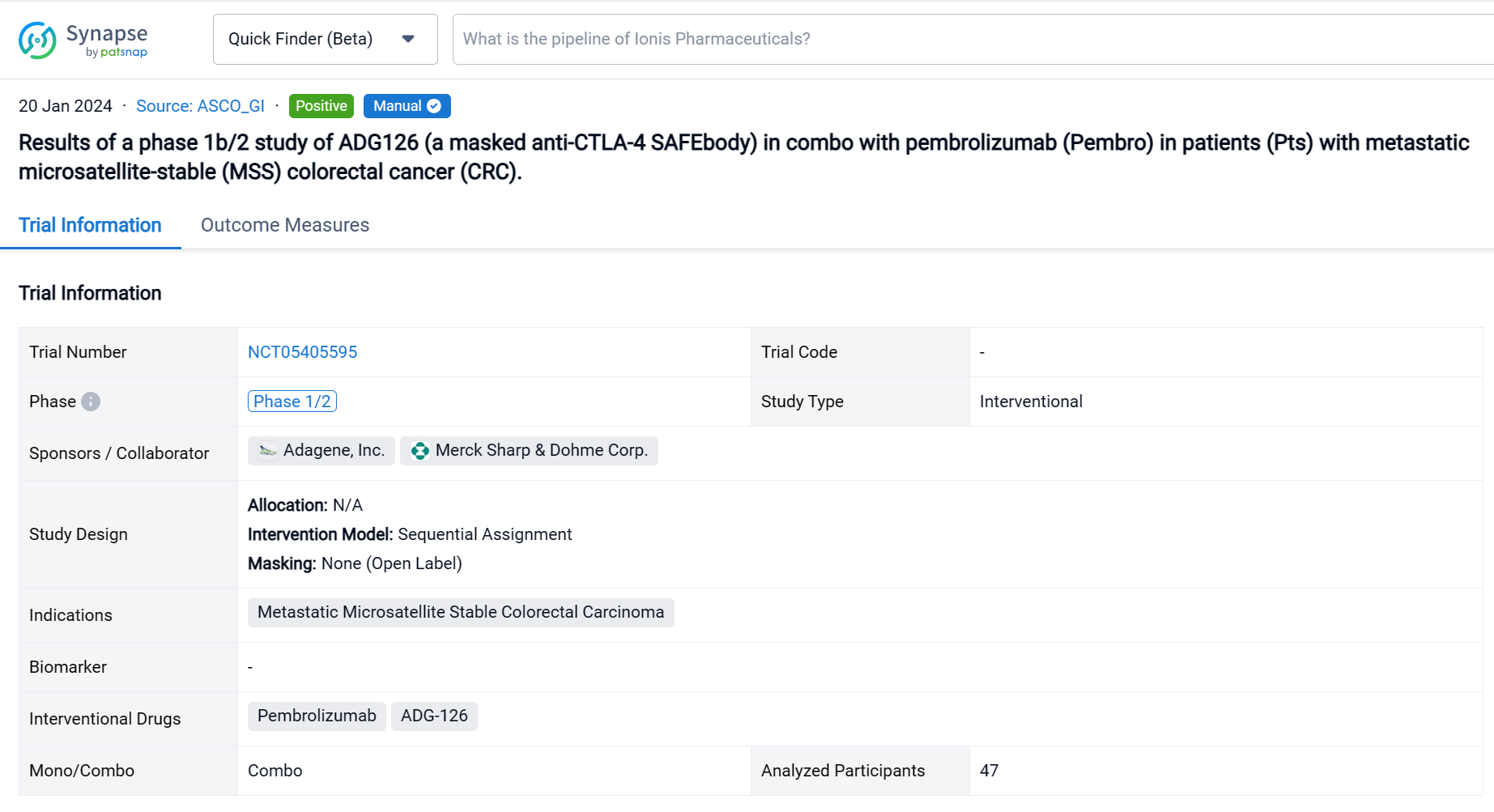
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
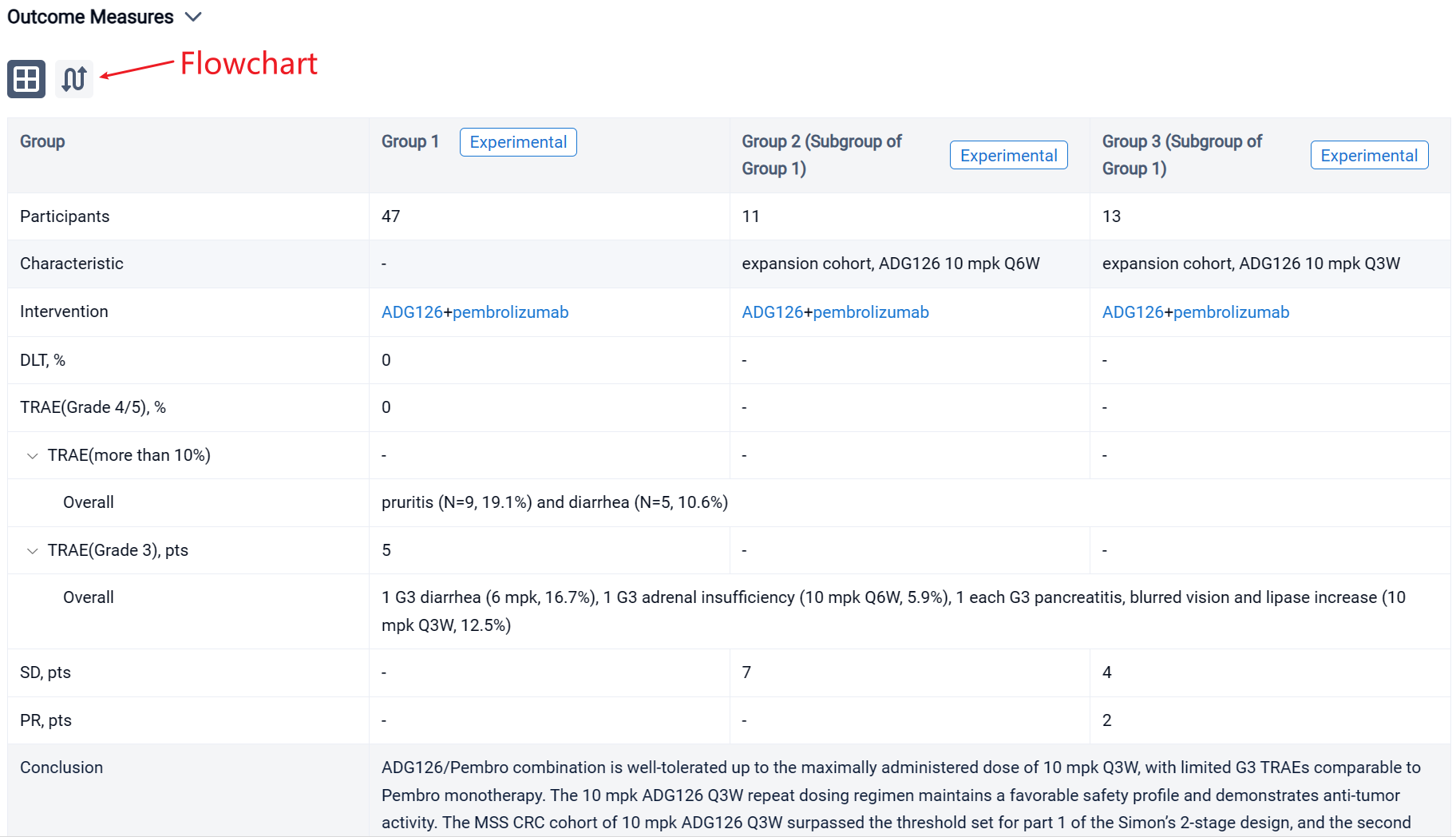
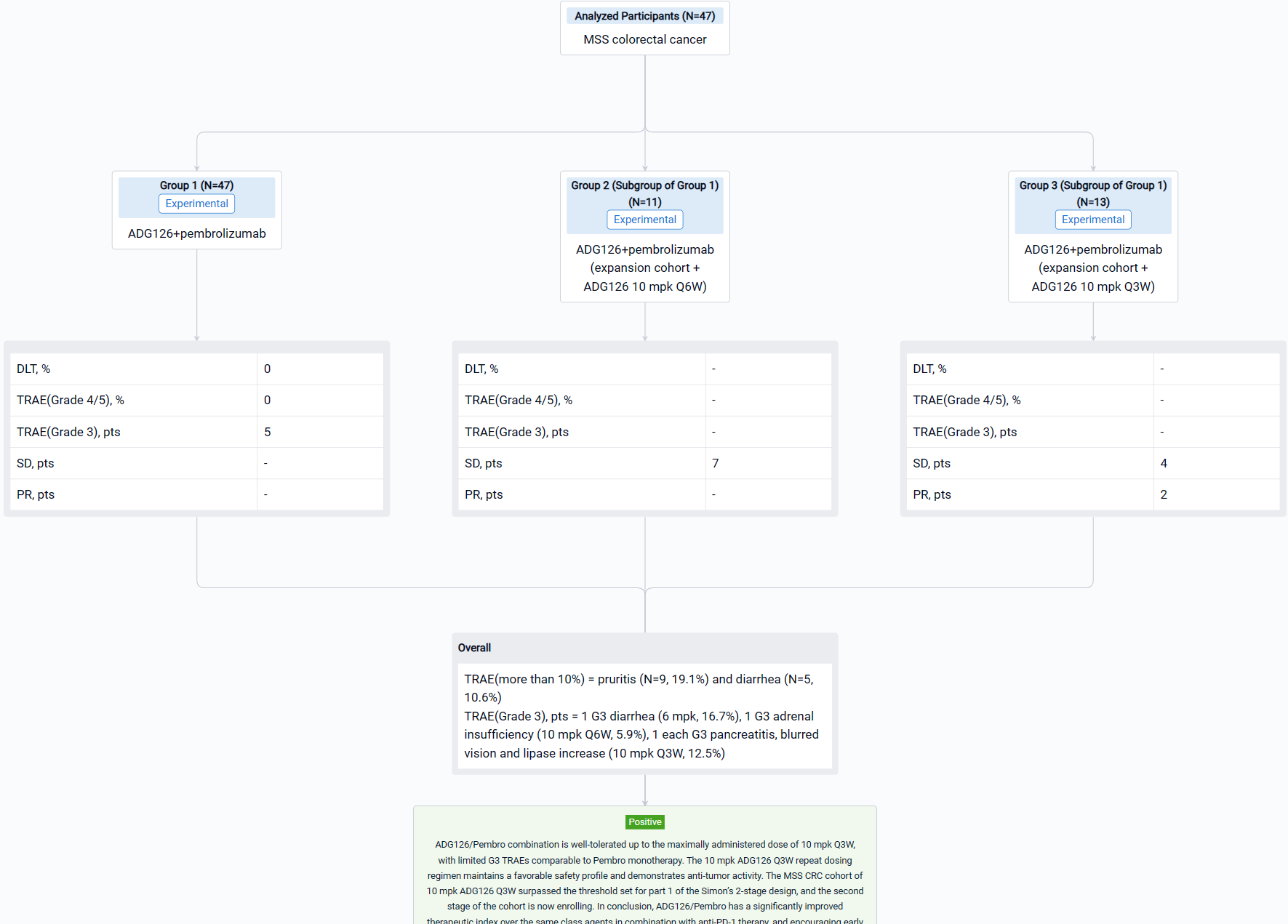
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!