MorphoSys to merge with Novartis under a new deal, total purchase price set at €2.7 billion in stock value
MorphoSys AG has officially declared that it has formed a strategic alliance through a Business Merger Contract with Novartis data42 AG and the principal entity, Novartis AG. This move follows Novartis' proactive proposal to launch a voluntary acquisition bid for the entire volume of MorphoSys' no-par value registered shares. The proposed transaction would involve a cash exchange, with shareholders receiving €68.00 for each share they hold.
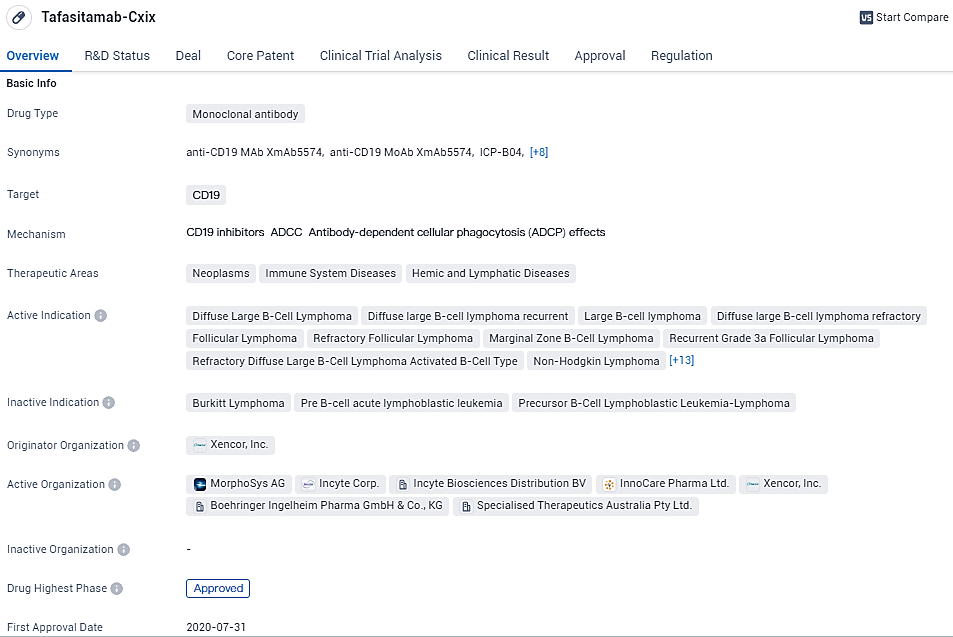
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In accordance with their Business Combination Agreement, Novartis is seeking to secure exclusive, global rights to advance and market two investigational compounds: pelabresib, a BET inhibitor, and tulmimetostat, an advanced dual inhibitor targeting both EZH2 and EZH1 enzymes, for various medical uses. Concurrently, MorphoSys has agreed to a deal with Incyte Corporation, divesting itself of all global rights to the drug tafasitamab.
MorphoSys has been collaborating with Incyte to enhance and bring tafasitamab to market. The unanimous consent for these business moves was granted by the boards overseeing MorphoSys.
Dr. Jean-Paul Kress, CEO of MorphoSys, expressed satisfaction regarding Incyte taking over the reins for tafasitamab. He emphasized that the decision was a strategic one, considering Novartis's proposal and the fruitful relationship with Incyte. Kress believes this approach serves MorphoSys and its stakeholders—including patients battling cancer—best.
The deal penned with Incyte allows the latter to gain exclusive global rights and bear all ongoing costs for tafasitamab's progression and marketing strategies, for a sum of US$ 25 million. Incyte's partnership with MorphoSys on tafasitamab dates back to 2020. Until this recent development, the U.S. saw tafasitamab co-promoted by MorphoSys and Incyte under the brand name Monjuvi (tafasitamab-cxix), while Incyte handled its promotion internationally under the brand name Minjuvi.
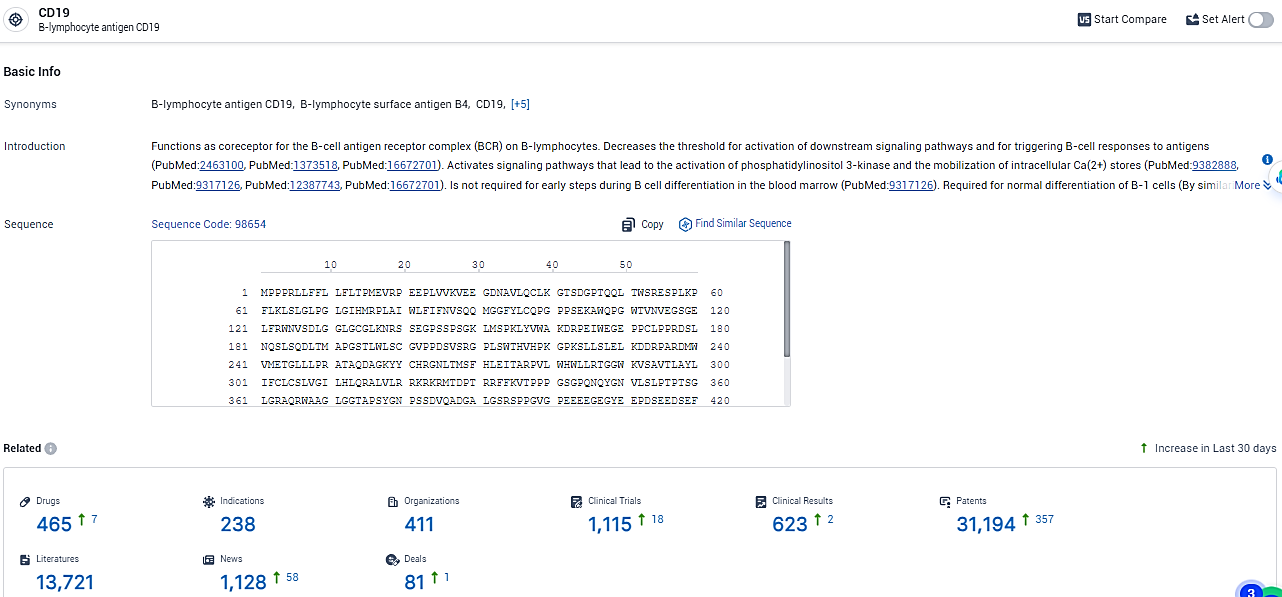
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 465 investigational drugs for the CD19 target, including 238 indications, 411 R&D institutions involved, with related clinical trials reaching 1115, and as many as 31194 patents.
Tafasitamab targets CD19 and is used in the treatment of various neoplasms and lymphatic diseases. Its approval in the United States and ongoing development in China highlight its potential as a therapeutic option for patients with different types of lymphomas and leukemias. The regulatory designations it has received further emphasize its importance in addressing unmet medical needs and expediting its availability to patients.