An analysis of Penpulimab's R&D progress and its clinical results presented at the 2023 ESMO_ASIA Annual Meeting
The Perioperative combinations including immune checkpoint inhibitors (ICIs) have shown encouraging results in patients with resectable locally advanced NSCLC, but the better treatment option needs to be explored further. On 2 Dec 2023, the latest clinical trial of Penpulimab-based combination neoadjuvant/adjuvant therapy for patients with resectable locally advanced non-small cell lung cancer was reported in 2023 ESMO_ASIA, demonstrating a promising treatment option.
Penpulimab's R&D Progress
Penpulimab is a monoclonal antibody drug that targets PD-1, a protein involved in regulating the immune system. The drug falls under the therapeutic areas of neoplasms, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, mouth and tooth diseases, otorhinolaryngologic diseases, and immune system diseases.
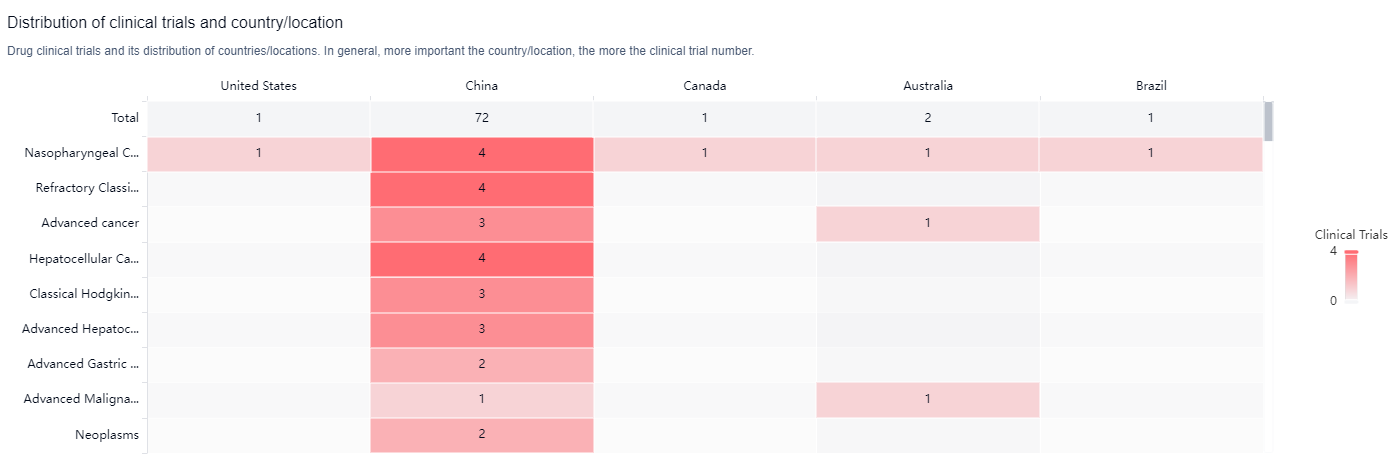
According to the Patsnap Synapse, Penpulimab has been developed by Akeso Biopharma Co., Ltd. and has received approvals globally. And the clinical trial distributions for Penpulimab are primarily in the United States, China and Canada. The key indication is Nasopharyngeal Carcinoma.
Detailed Clinical Result of Penpulimab
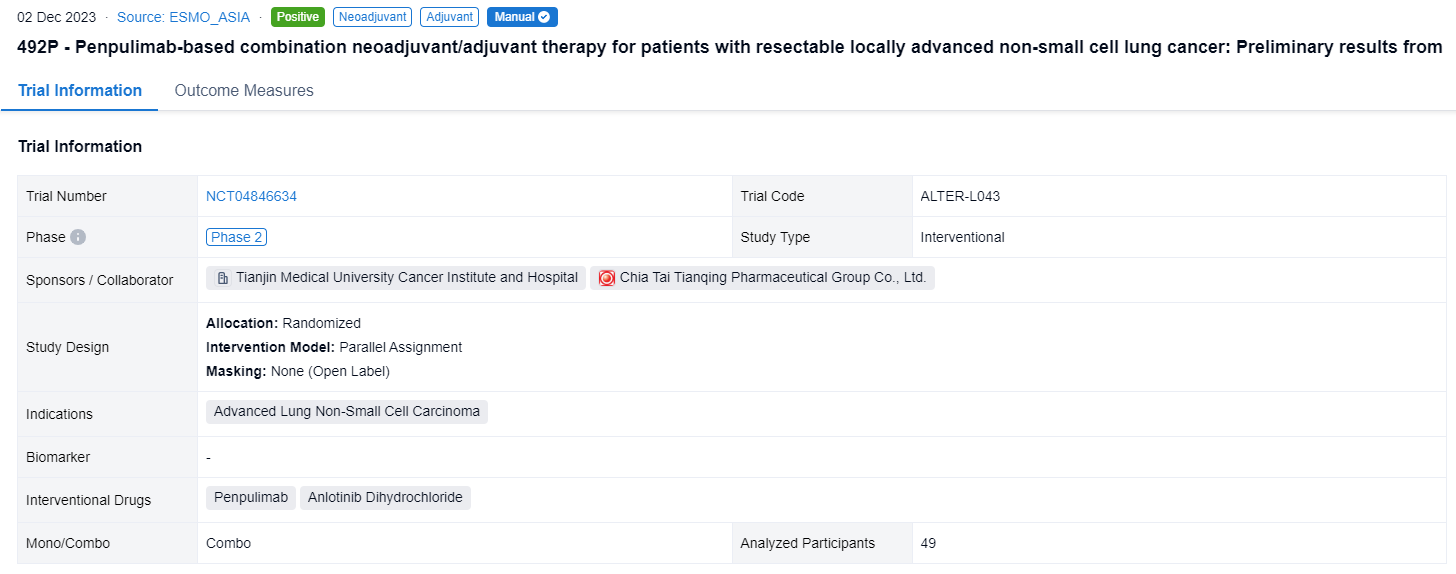
This randomized, parallel assignment, open-labeled clinical trial (NCT04846634) was designed to investigate the efficacy and safety of Penpulimab-based combination neoadjuvant/adjuvant therapy for non-small cell lung cancer.
In this study, eligible patients (pts) without driver gene mutations, resectable clinical stage IIB-IIIB (N2) NSCLC, were randomized 1:1:1 to receive one of the three regimens in 21-day cycle: Penpulimab (200mg, iv, day 1) + chemotherapy + Anlotinib (12mg, po, day 1-14) (Arm A) or Penpulimab (200mg, iv, day 1) + chemotherapy (Arm B) or Penpulimab (200mg, iv, day 1) + Anlotinib (12mg, po, day 1-14) (Arm C) for 3-4 cycles before surgery, followed by adjuvant therapy of Penpulimab + Anlotinib (Arm A, C) or Penpulimab monotherapy (Arm B) for a year at most. Primary endpoint was major pathological response (MPR) rate, secondary endpoints were objective response rate (ORR), pathologic complete response (pCR), event-free survival (EFS), 1 year EFS rate, overall survival (OS) and safety.
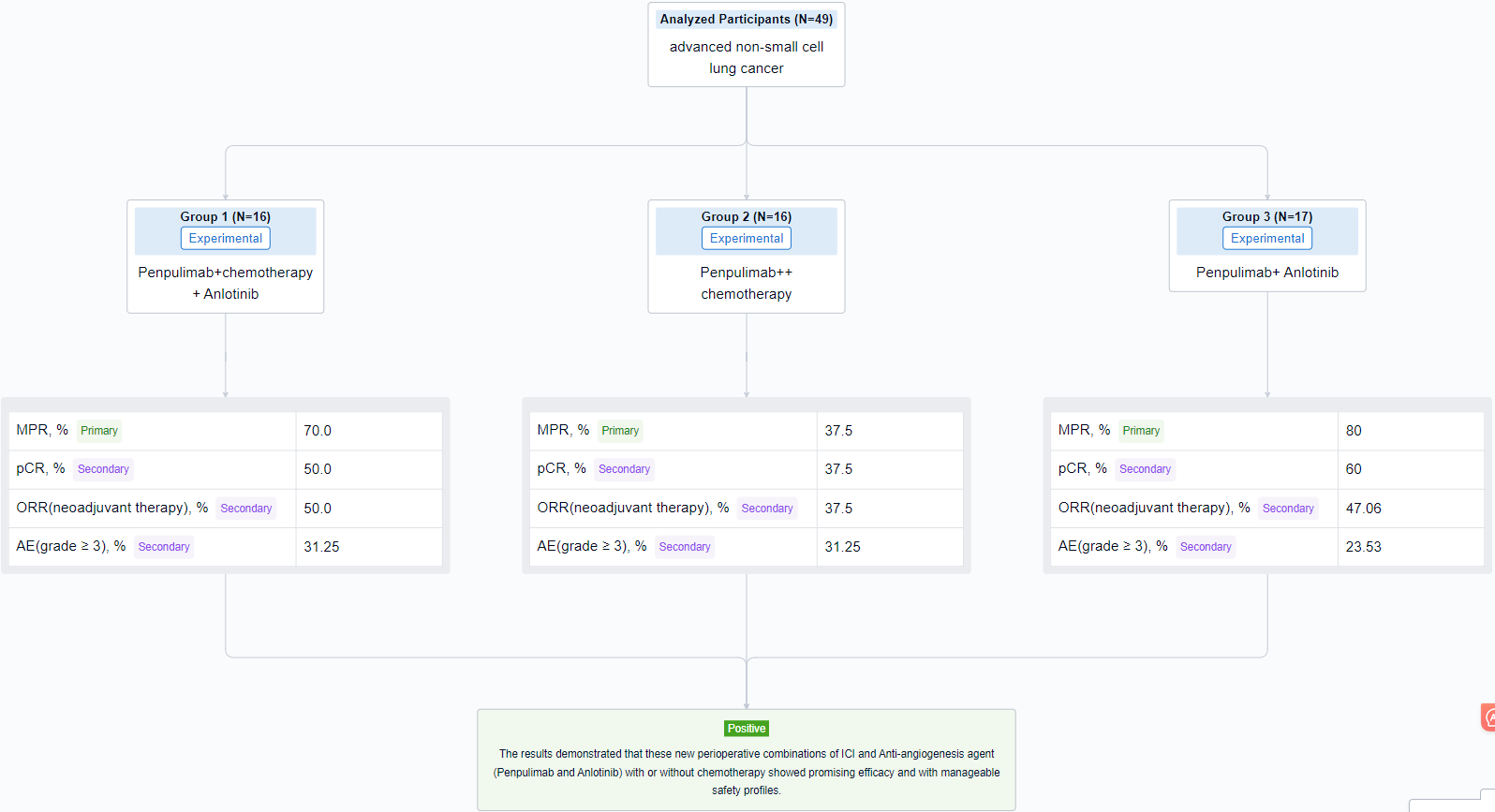
The result showed that from December, 2021 to August, 2023, 49 pts were randomized to Arm A (n=16) or Arm B (n=16) or Arm C (n=17). At data cutoff (Aug 3, 2023), median follow-up was 5.3 months. Definitive surgery rates in Arm A/B/C were 87.5% vs 87.5% vs 76.5% respectively. The MPR rates were 70.0% vs 37.5% vs 80% in the three arms, and 50.0% vs 37.5% vs 60% pts showed pCR respectively. ORR of neoadjuvant therapy was 50.0% vs 37.5% vs 47.06% in the three arms. The incidence of grade ≥ 3 adverse events (AEs) were 31.25% vs 31.25% vs 23.53% respectively. There is no fatal AE related to Penpulimab or Anlotinib.
It can be concluded that these new perioperative combinations of ICI and Anti-angiogenesis agent (Penpulimab and Anlotinib) with or without chemotherapy showed promising efficacy and with manageable safety profiles.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
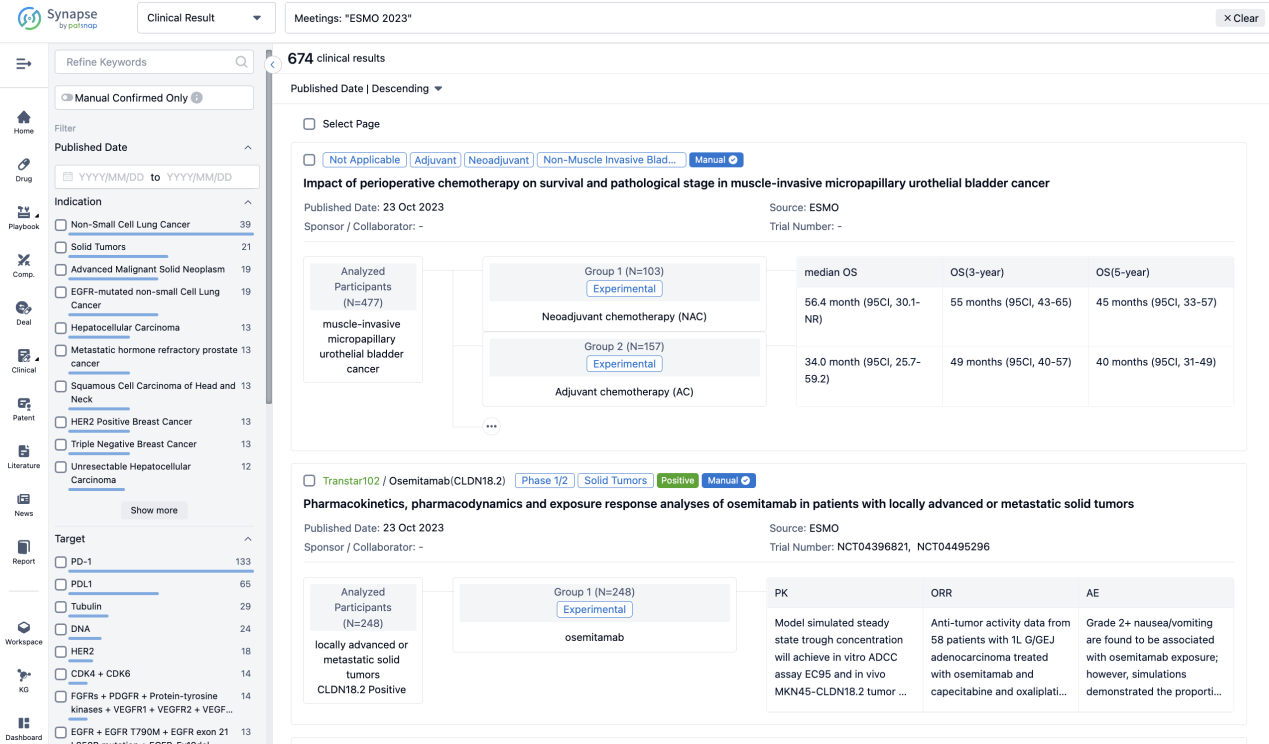
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
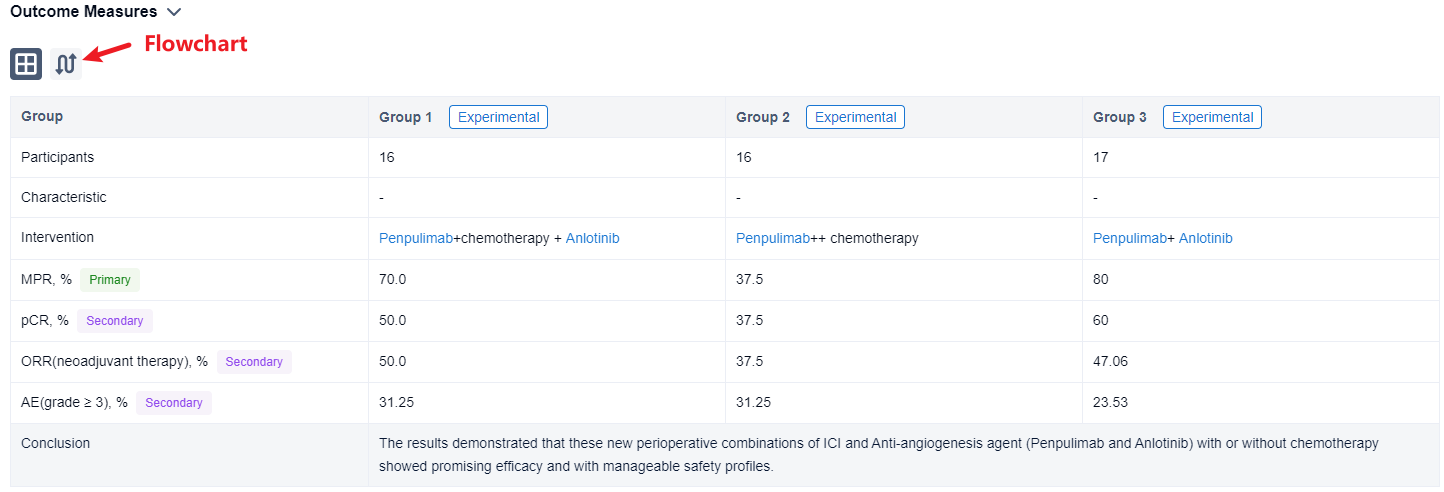

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!