An In-depth Analysis of cefroxadine's R&D Progress and Mechanism of Action on Drug Target
Cefroxadine's R&D Progress
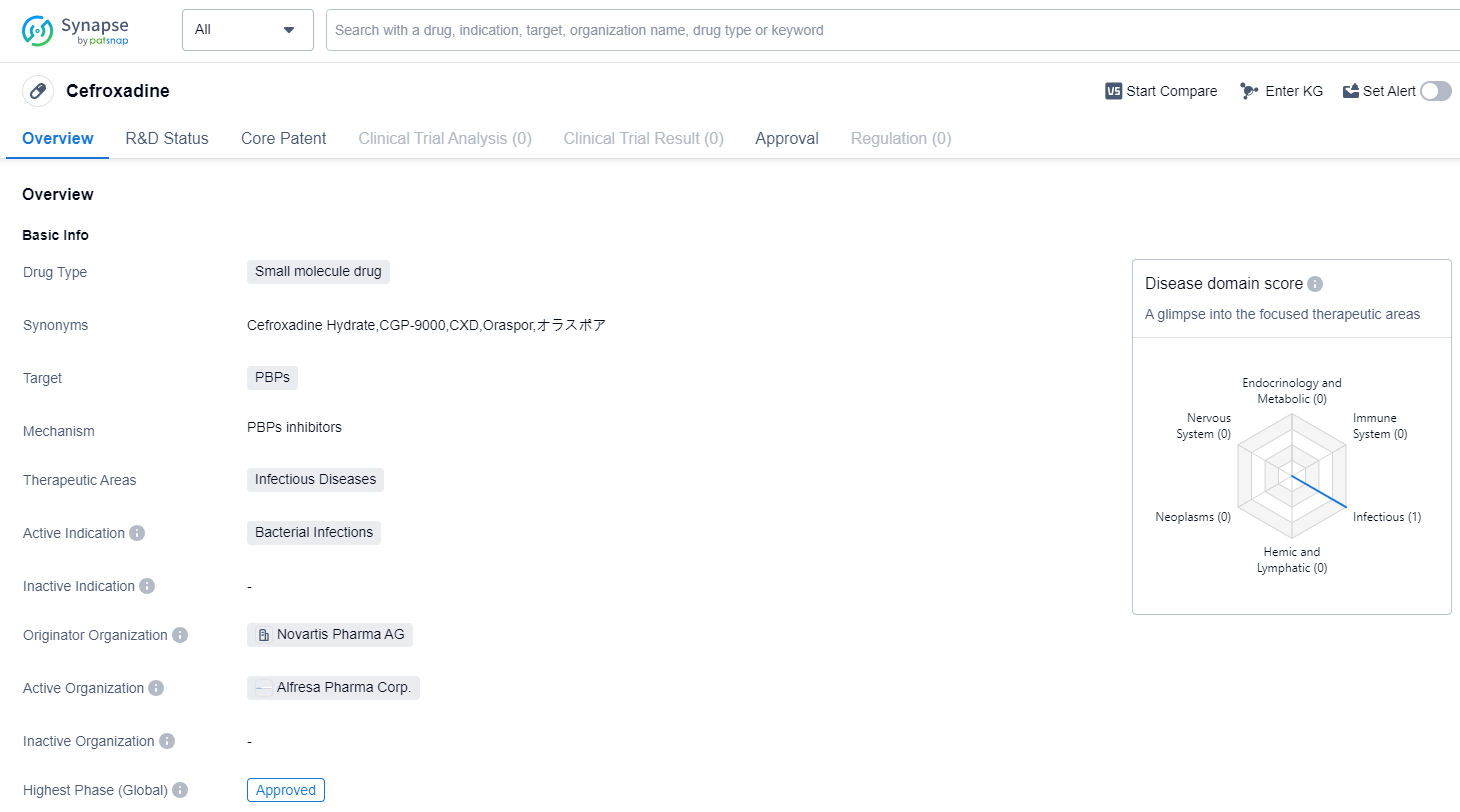
Cefroxadine is a small molecule drug that falls under the therapeutic area of infectious diseases. It is primarily used for the treatment of bacterial infections. The drug targets PBPs (penicillin-binding proteins) to exert its therapeutic effects. Cefroxadine was first approved in Japan in December 1982. The highest R&D phase of this drug is approved.
The originator organization of Cefroxadine is Novartis Pharma AG, a renowned pharmaceutical company known for its contributions to the healthcare industry. Novartis Pharma AG has played a significant role in the development and commercialization of this drug.
As a small molecule drug, Cefroxadine is designed to interact with specific targets, in this case, PBPs. PBPs are enzymes involved in the synthesis of bacterial cell walls. By targeting these proteins, Cefroxadine disrupts the formation of the bacterial cell wall, leading to the inhibition of bacterial growth and ultimately the eradication of the infection.
Cefroxadine's approval in Japan in 1982 indicates that it has undergone rigorous testing and evaluation to demonstrate its safety and efficacy. The drug has successfully met the regulatory requirements and has been deemed suitable for use in treating bacterial infections.
The approval of Cefroxadine in Japan suggests that it has demonstrated positive clinical outcomes and has met the necessary standards for patient safety. This approval also signifies that the drug has undergone extensive clinical trials and has been proven effective in treating bacterial infections.
Given the information provided, Cefroxadine has a long history of use, with its first approval dating back to 1982. This indicates that it has been in the market for several decades and has likely established a solid reputation for its efficacy and safety profile.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for cefroxadine: PBPs inhibitors
PBPs inhibitors are a type of drugs that target and inhibit the activity of penicillin-binding proteins (PBPs). PBPs are enzymes found in the cell wall of bacteria and are involved in the synthesis of peptidoglycan, a crucial component of the bacterial cell wall. By inhibiting PBPs, these inhibitors disrupt the formation of the bacterial cell wall, leading to cell lysis and ultimately killing the bacteria.
From a biomedical perspective, PBPs inhibitors are important in the field of antimicrobial therapy. They are commonly used to treat bacterial infections caused by susceptible bacteria. By specifically targeting PBPs, these inhibitors selectively affect bacterial cells while sparing human cells, as human cells do not have peptidoglycan in their cell walls. This selective action makes PBPs inhibitors effective antibiotics with a relatively low risk of toxicity to the host.
It is worth noting that different classes of PBPs inhibitors exist, such as beta-lactam antibiotics (e.g., penicillins, cephalosporins) and glycopeptide antibiotics (e.g., vancomycin). These inhibitors have varying mechanisms of action and spectra of activity against different bacteria. The choice of PBPs inhibitor depends on the specific bacterial infection being treated and the susceptibility of the bacteria to the drug.
Drug Target R&D Trends for cefroxadine
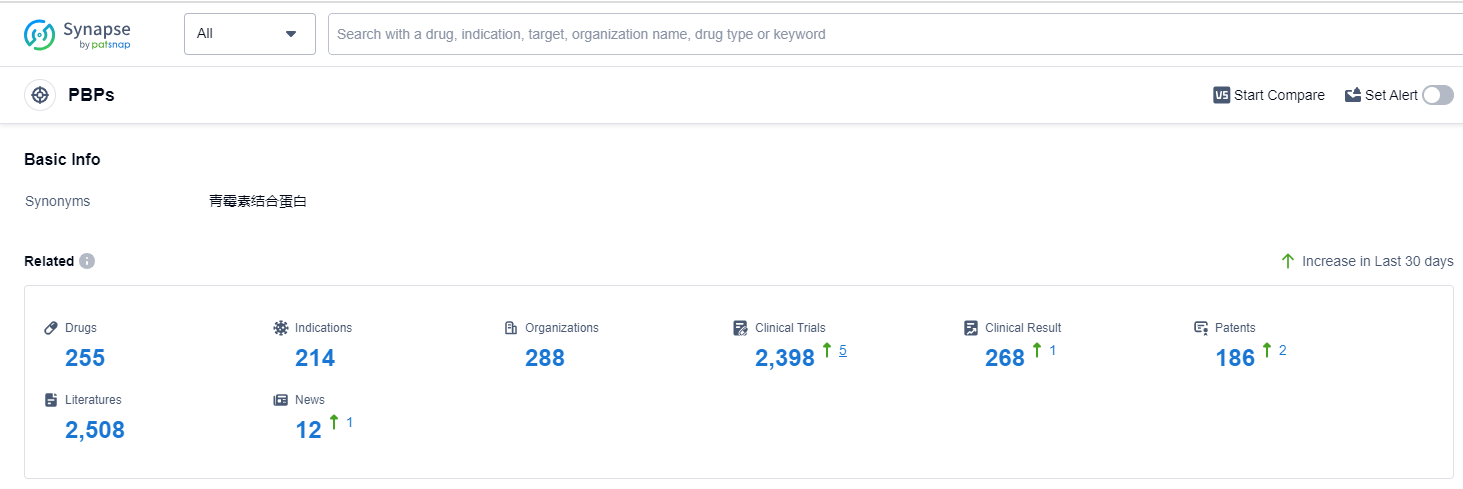
PBPs, or Penicillin-Binding Proteins, play a crucial role in the human body. These proteins are found in the cell walls of bacteria and are responsible for the synthesis and maintenance of the peptidoglycan layer, a vital component of bacterial cell walls. PBPs act as targets for antibiotics, particularly beta-lactam antibiotics like penicillin, by binding to them and inhibiting the enzymes that cross-link the peptidoglycan strands. This disruption weakens the bacterial cell wall, leading to cell lysis and ultimately bacterial death. Understanding the role of PBPs is essential in developing effective antibiotics and combating bacterial infections.
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 255 PBPs drugs worldwide, from 288 organizations, covering 214 indications, and conducting 2398 clinical trials.
The analysis of the target PBPs in the pharmaceutical industry reveals a competitive landscape with several companies actively developing drugs. Pfizer Inc., Meiji Holdings Co., Ltd., Takeda Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Merck & Co., Inc. are the companies growing fastest under the current target. The indications for the drugs under the target PBPs primarily focus on bacterial infections and related diseases. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly, indicating potential for innovative treatments. China, Japan, the United States, and the European Union are the countries/locations developing fastest under the target PBPs, with China showing significant progress. Overall, the target PBPs present opportunities for growth and innovation in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cefroxadine is a small molecule drug developed by Novartis Pharma AG for the treatment of bacterial infections. It targets PBPs and has been approved in Japan since 1982. The drug's approval and long history of use suggest its effectiveness and safety in treating bacterial infections.