IASO Bio showcases Updated Long-Term Follow-Up information for BCMA CAR-T FUCASO®
IASO Bio, a biotech corporation specialized in the identification, development, production, and distribution of groundbreaking cell therapies and antibody solutions, along with Innovent Biologics, Inc., a leading global biopharmaceutical enterprise devoted to producing and selling top-notch medicinal treatments for conditions such as cancer, metabolic disorders, autoimmune diseases, ophthalmologic conditions and other significant illnesses, have unveiled revised long-term follow-up statistics derived from a couple of studies conducted on BCMA CAR-T.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
These studies focused on the outcomes of the Phase 1b/2 study carried out on patients who had relapsed/refractory multiple myeloma and were undergoing treatment with Equecabtagene Autoleucel.
A model designed to estimate the likelihood of lengthy recovery from thrombocytopenia in patients suffering from relapsed/refractory multiple myeloma after undergoing anti-BCMA CAR-T treatment will be presented at the 2023 International Myeloma Society Annual Meeting in Vienna, taking place from September 27-30.
CT103A, known as Equecabtagene Autoleucel, is a type of chimeric antigen receptor T-cell therapy made entirely of human-made B-cell maturation antigen-targeting single-chain fragment variable antibody. The latest findings showed a long-term follow-up effectiveness, safety, and the continuity of the phase 1b/2 study completed in 14 clinical sites across China.
Patients with RRMM who had received anti-B cell maturation antigen chimeric antigen receptor-T cell therapy commonly face persistent thrombocytopenia, a hematologic toxicity. This presentation showcases a model that was developed to distinguish between patients at high and low risk for prolonged thrombocytopenia.
The model was devised following an analysis of the base-level clinical attributes of the study's participants, including platelets, C reaction protein, plasma cells present in the marrow, and Ferritin, which was found to contribute significantly to thrombocytopenia recovery. Patients who have a model score of more than 5 post-infusion are at a higher risk of prolonged thrombocytopenia.
"Multiple myeloma is a malignant blood disease that's highly prevalent and relapse and resistance are almost always unavoidable after the existing treatment,” Prof. Lugui Qiu, MD, one of the leading investigators from the Chinese Academy of Medical Science Hematology Hospital, said. "It is critical to find ways to ensure longer-lasting treatments. Equecabtagene Autoleucel, a fully human targeted BCMA CAR-T injection, has proven its ability to have long-term effects and generate a prolonged response."
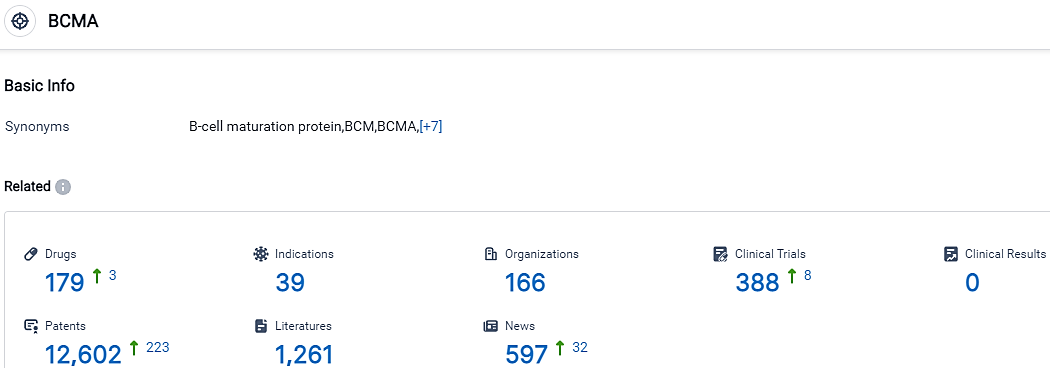
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 8, 2023, there are 179 investigational drugs for the BCMA target, including 39 indications,166 R&D institutions involved, with related clinical trials reaching 388,and as many as 12602 patents.
Equecabtagene Autoleucel represents a significant advancement in the treatment of multiple diseases, particularly in the field of oncology. Its approval in China and global markets, along with its regulatory designations, highlight its potential to improve patient outcomes and address unmet medical needs.