An In-depth Analysis of Nebivolol hydrochloride's R&D Progress
Nebivolol hydrochloride's R&D Progress
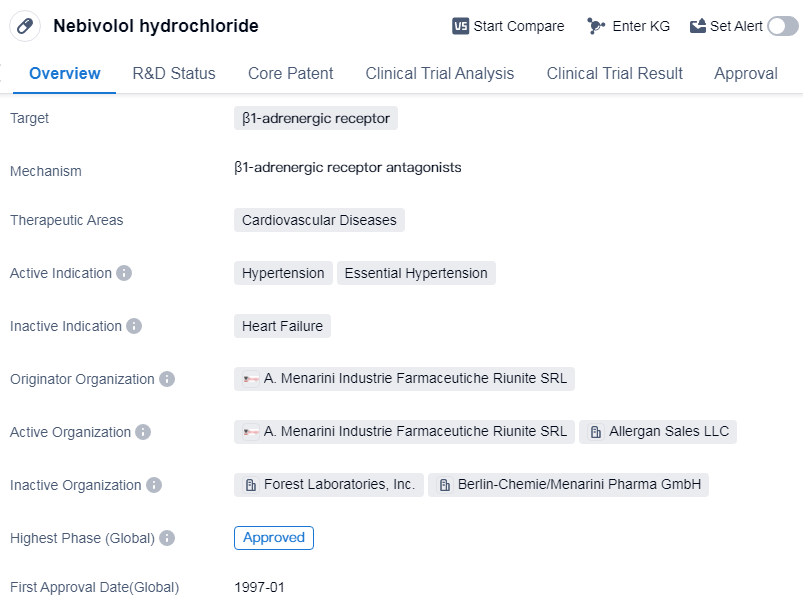
Nebivolol hydrochloride is a small molecule drug that targets the β1-adrenergic receptor. It is primarily used in the treatment of cardiovascular diseases, specifically hypertension and essential hypertension. The drug was first approved in Germany in January 1997 and has since gained approval in other countries globally.
The originator organization of Nebivolol hydrochloride is A. Menarini Industrie Farmaceutiche Riunite SRL. This pharmaceutical company played a crucial role in the development and commercialization of the drug. Nebivolol hydrochloride has reached the highest phase of development, which is the approved stage, in the global market. This indicates that it has successfully undergone rigorous testing and evaluation to demonstrate its safety and efficacy.
In China, Nebivolol hydrochloride is currently in phase 3 of development, suggesting that it is undergoing clinical trials to assess its effectiveness and safety in the Chinese population. This phase is a critical step towards obtaining regulatory approval in China.
The therapeutic areas of Nebivolol hydrochloride are focused on cardiovascular diseases, particularly hypertension. Hypertension, also known as high blood pressure, is a prevalent condition that affects a significant portion of the global population. Essential hypertension refers to high blood pressure with no identifiable cause, making it a primary concern for healthcare providers.
Nebivolol hydrochloride acts by selectively blocking the β1-adrenergic receptor, which is involved in regulating heart rate and blood pressure. By targeting this receptor, the drug helps to reduce blood pressure and improve cardiovascular function.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nebivolol hydrochloride: β1-adrenergic receptor antagonists
β1-adrenergic receptor antagonists, also known as beta-1 blockers, are a class of drugs that block the action of the β1-adrenergic receptors in the body. These receptors are part of the sympathetic nervous system and are primarily found in the heart. By blocking the β1-adrenergic receptors, these drugs reduce the effects of the stress hormone adrenaline (epinephrine) on the heart.
From a biomedical perspective, β1-adrenergic receptor antagonists are commonly used in the treatment of various cardiovascular conditions. They work by decreasing the heart rate and reducing the force of contraction of the heart muscle, thereby lowering blood pressure and reducing the workload on the heart. This class of drugs is often prescribed to manage conditions such as hypertension (high blood pressure), angina (chest pain), and heart failure.
By blocking the β1-adrenergic receptors specifically, these drugs selectively target the receptors in the heart, minimizing the impact on other β-adrenergic receptors found in other tissues. This selectivity helps to reduce potential side effects and allows for more precise control over heart function.
It is important to note that β1-adrenergic receptor antagonists should be used under the supervision of a healthcare professional, as they may interact with other medications and can have side effects such as fatigue, dizziness, and sexual dysfunction. The specific drug chosen within this class may vary based on individual patient factors and the specific condition being treated.
Drug Target R&D Trends for Nebivolol hydrochloride
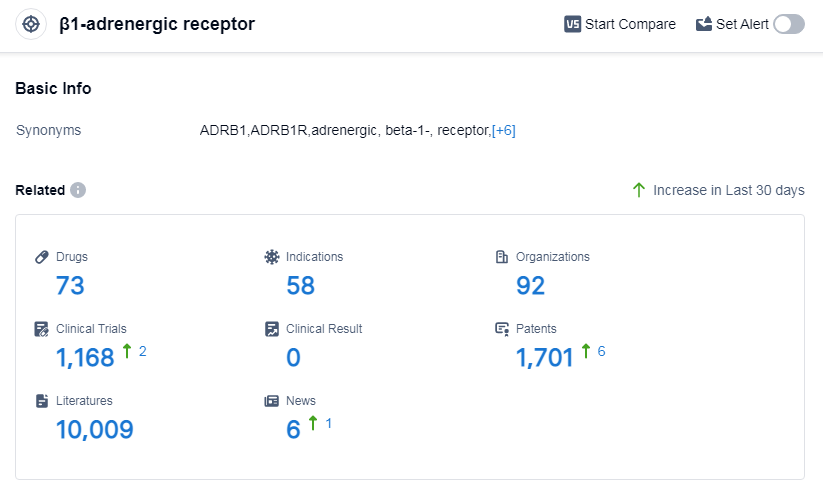
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 73 β1-adrenergic receptor drugs worldwide, from 92 organizations, covering 58 indications, and conducting 1168 clinical trials.
The analysis of the target β1-adrenergic receptor reveals a competitive landscape with multiple companies actively involved in its research and development. AstraZeneca PLC, Sanofi, Novartis AG, GSK Plc, and A. Menarini Industrie Farmaceutiche Riunite SRL are the companies growing fastest under this target. The most common indications for drugs targeting this receptor include hypertension, angina pectoris, and heart failure. Small molecule drugs are progressing most rapidly, indicating intense competition in the development of innovative drugs. China, the United States, and Japan are the countries developing fastest under this target, with China showing significant progress. Overall, the target β1-adrenergic receptor presents opportunities for further research and development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nebivolol hydrochloride is a small molecule drug developed by A. Menarini Industrie Farmaceutiche Riunite SRL. It is primarily used in the treatment of hypertension and essential hypertension. The drug has gained regulatory approval in multiple countries globally, with its first approval in Germany in 1997. It is currently in phase 3 of development in China.