An In-depth Analysis of Pimozide's R&D Progress and Mechanism of Action on Drug Target
Pimozide's R&D Progress
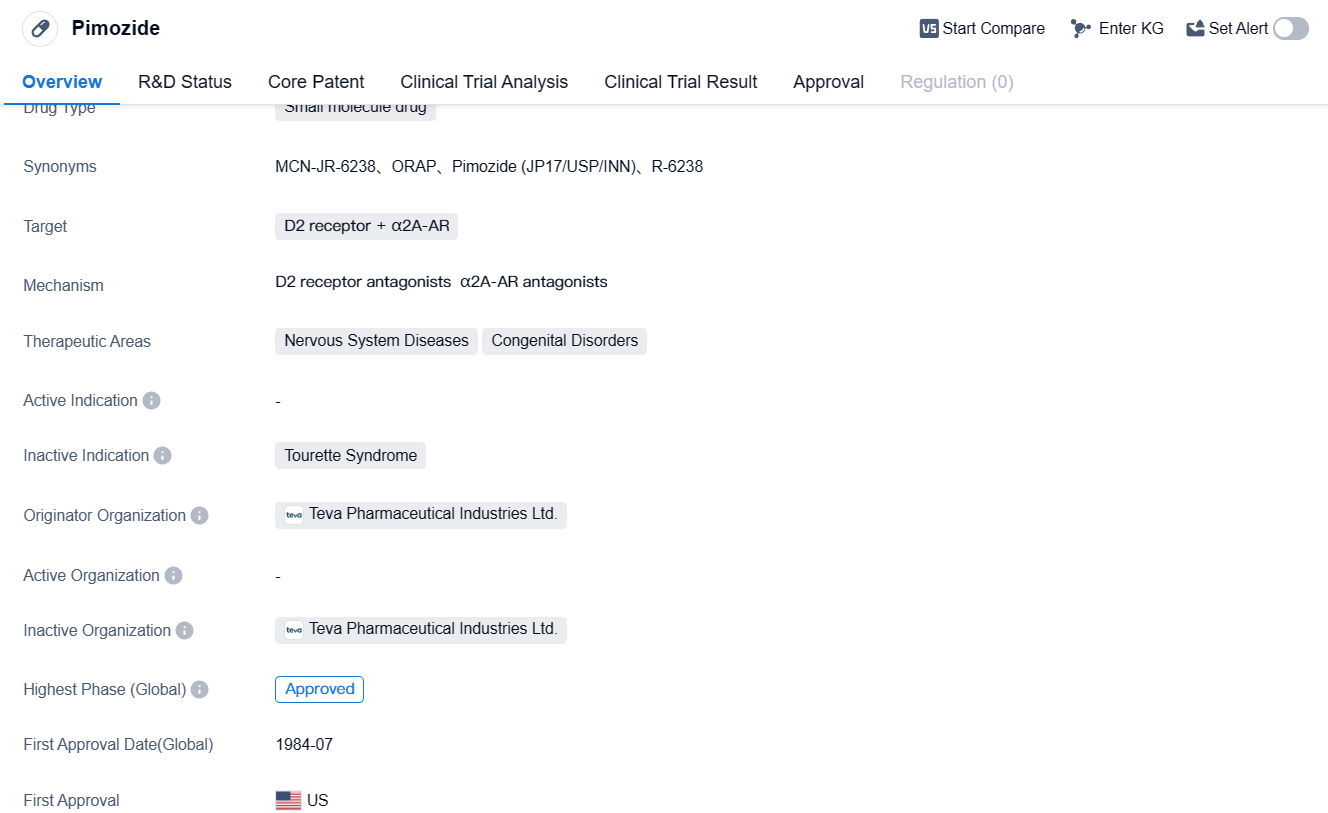
Pimozide is a small molecule drug that primarily targets the D2 receptor and α2A-AR in the field of biomedicine. It is used for the treatment of nervous system diseases and congenital disorders.
Pimozide is developed by Teva Pharmaceutical Industries Ltd., a well-known pharmaceutical organization. As a small molecule drug, it is designed to interact with specific receptors in the body, namely the D2 receptor and α2A-AR. These receptors are believed to play a crucial role in the regulation of various functions in the nervous system.
The therapeutic areas of Pimozide include nervous system diseases and congenital disorders. Nervous system diseases encompass a wide range of conditions that affect the brain, spinal cord, and nerves, such as schizophrenia, Tourette syndrome, and bipolar disorder. Congenital disorders, on the other hand, refer to conditions that are present at birth and may affect the development and functioning of various body systems.
Pimozide has undergone rigorous testing and evaluation to reach its current highest phase of approval globally. The drug's first approval in the United States in 1984 signifies its long-standing presence in the market and its established track record.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pimozide: D2 receptor antagonists and α2A-AR antagonists
D2 receptor antagonists are a class of drugs that block the activity of the D2 receptors in the brain. D2 receptors are a subtype of dopamine receptors, which are involved in regulating various functions in the central nervous system. By blocking D2 receptors, these antagonists can modulate the effects of dopamine, a neurotransmitter involved in reward, motivation, and movement control. D2 receptor antagonists are commonly used in the treatment of psychiatric disorders such as schizophrenia, as they help to reduce the symptoms associated with excessive dopamine activity.
α2A-AR antagonists, on the other hand, refer to alpha-2A adrenergic receptor antagonists. Alpha-2A adrenergic receptors are a type of receptor found in the central and peripheral nervous systems that bind to norepinephrine, a neurotransmitter involved in the regulation of blood pressure, attention, and stress response. By antagonizing or blocking these receptors, α2A-AR antagonists can enhance the release of norepinephrine, leading to increased arousal and improved cognitive function. These antagonists have potential applications in the treatment of attention deficit hyperactivity disorder (ADHD) and cognitive impairment associated with certain neurological conditions.
Drug Target R&D Trends for Pimozide
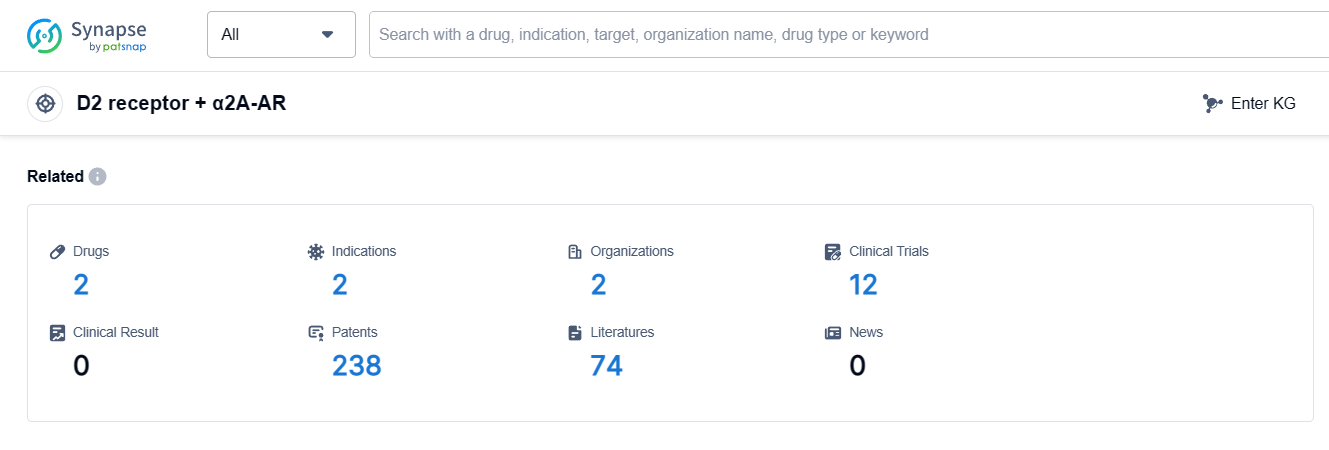
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 2 D2 receptor + α2A-AR drugs worldwide, from 2 organizations, covering 2 indications, and conducting 12 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for the target D2 receptor + α2A-AR is characterized by companies in the inactive phase of development, with Sumitomo Chemical Co., Ltd. and Teva Pharmaceutical Industries Ltd. being the leading companies. There are no drugs approved for relevant indications under this target, indicating that further research and development is required. Small molecule drugs are the primary focus in the development efforts, and the United States has shown the most progress in terms of approved drugs and drugs in the inactive phase. However, more comprehensive data and analysis are needed to fully understand the competitive landscape and future development prospects for the target D2 receptor + α2A-AR in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Based on the given information, it can be concluded that Pimozide is a small molecule drug developed by Teva Pharmaceutical Industries Ltd. It targets the D2 receptor and α2A-AR and is approved for the treatment of nervous system diseases and congenital disorders. Its first approval in the United States dates back to July 1984, indicating its long history in the market.