An Overview of AbbVie 's Drug Pipelines|R&D Progress|Drug target
AbbVie, Inc. is a pharmaceutical organization that was founded in 2013 and is based in Illinois, United States. Since its establishment, AbbVie has been actively involved in the development of drugs and therapies across various therapeutic areas in the field of biomedicine.
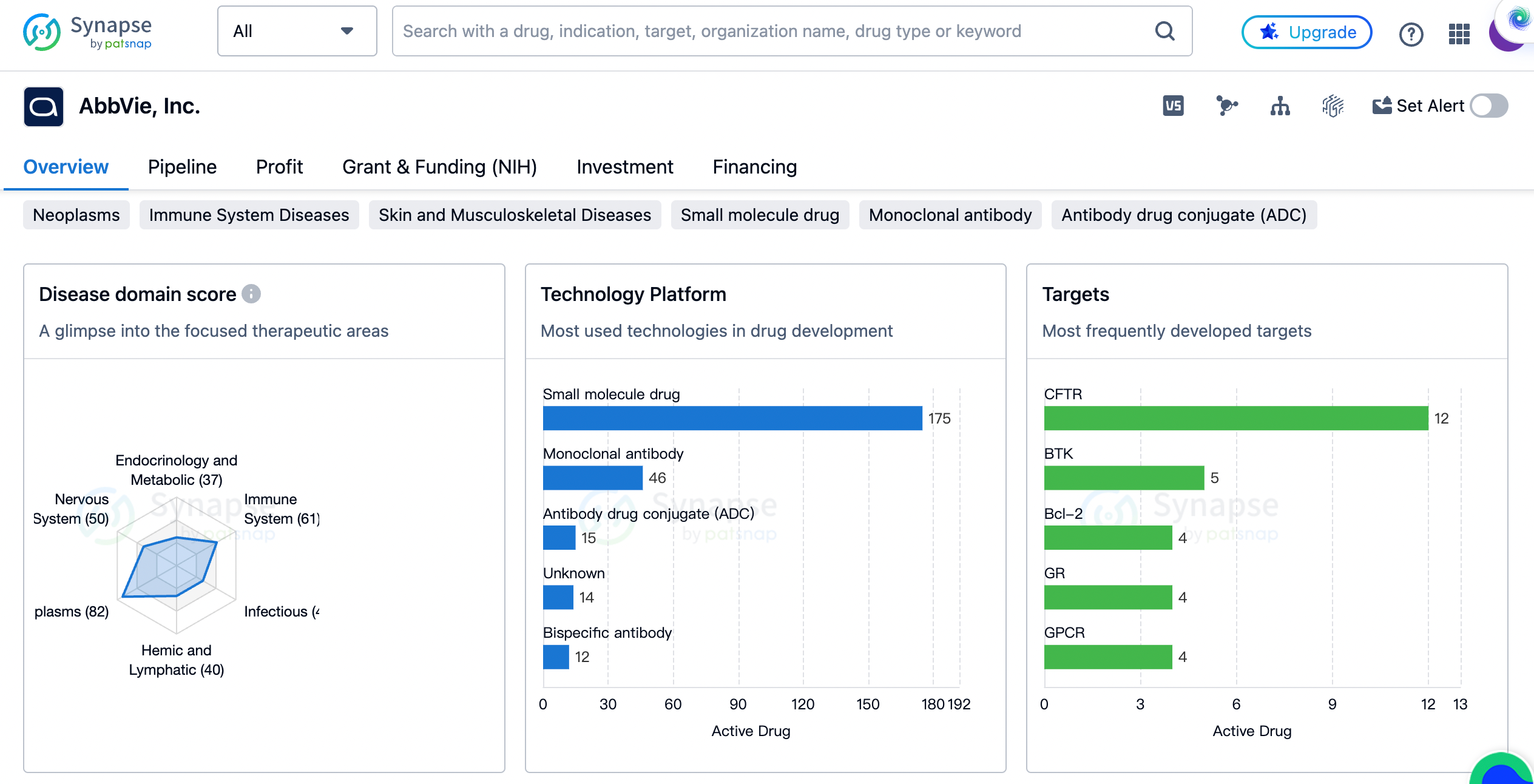
AbbVie has a diverse portfolio of drugs that target different therapeutic areas. The organization has the highest drug count in the field of Neoplasms, with 82 drugs developed for the treatment of various types of cancers. This indicates AbbVie's strong focus on oncology and its commitment to addressing the unmet medical needs of cancer patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of AbbVie.
Following Neoplasms, AbbVie has developed a significant number of drugs for Digestive System Disorders (64 drugs), Immune System Diseases (61 drugs), Skin and Musculoskeletal Diseases (55 drugs), and Other Diseases (53 drugs). These therapeutic areas highlight AbbVie's dedication to developing treatments for a wide range of medical conditions, including gastrointestinal disorders, autoimmune diseases, and dermatological conditions.
AbbVie has also made substantial contributions to the treatment of Respiratory Diseases (51 drugs), Nervous System Diseases (50 drugs), Eye Diseases (45 drugs), Hemic and Lymphatic Diseases (40 drugs), Infectious Diseases (40 drugs), Urogenital Diseases (38 drugs), Endocrinology and Metabolic Disease (37 drugs), Congenital Disorders (31 drugs), and Cardiovascular Diseases (29 drugs). This demonstrates AbbVie's commitment to addressing the healthcare needs of patients across various therapeutic areas.
The most frequently developed targets by AbbVie
The CFTR target has the highest drug count, with 12 drugs developed. CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) is a protein involved in the regulation of chloride ion transport across epithelial cells. AbbVie's focus on CFTR indicates its dedication to developing treatments for cystic fibrosis, a genetic disorder that affects the lungs and other organs.
Other frequently developed targets by AbbVie include BTK (Bruton's Tyrosine Kinase), Bcl-2 (B-cell lymphoma 2), GR (Glucocorticoid Receptor), GPCR (G-protein coupled receptor), SERT (Serotonin Transporter), SNAP25 (Synaptosomal-Associated Protein 25), FXR (Farnesoid X Receptor), EGFR (Epidermal Growth Factor Receptor), mAChRs (Muscarinic Acetylcholine Receptors), CD40 (Cluster of Differentiation 40), NMDA receptor (N-Methyl-D-Aspartate receptor), NS3/NS4A (Non-structural protein 3/Non-structural protein 4A), BRD4 (Bromodomain-containing protein 4), SARS-CoV-2 S protein (Spike protein of Severe Acute Respiratory Syndrome Coronavirus 2), Prostanoid receptor, Tumor-associated antigen, PBPs (Penicillin-Binding Proteins), SV2A (Synaptic Vesicle Glycoprotein 2A), and NS5B polymerase (Non-structural protein 5B polymerase).
These targets represent a wide range of biological pathways and molecular mechanisms that AbbVie is actively exploring for the development of novel therapies. The diversity of targets reflects AbbVie's commitment to innovation and its pursuit of new treatment options for various diseases.
An overview of AbbVie's pipeline
Here’s an overview of AbbVie's pipeline, indicating the number of drugs in different phases of development until August 30, 2023. The pipeline includes drugs in various stages, starting from the Discovery phase (10 drugs) and progressing through Preclinical (28 drugs), IND (Investigational New Drug) stage (1 drug), IND Approval (0 drugs), Phase 1 (72 drugs), Phase 2 (50 drugs), Phase 3 (20 drugs), NDA/BLA (New Drug Application/Biologics License Application) stage (2 drugs), and Approved (100 drugs). Additionally, there are 370 drugs categorized under "Other," which could include drugs in early stages of development or those with uncertain timelines.
The significant number of drugs in the pipeline reflects AbbVie's commitment to research and development, as well as its focus on advancing potential therapies through clinical trials and regulatory approval processes. The pipeline demonstrates AbbVie's dedication to bringing new treatments to market and addressing unmet medical needs across various therapeutic areas.
In conclusion, AbbVie, Inc. is a pharmaceutical organization founded in 2013 and based in Illinois, United States. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a strong focus on Neoplasms, Digestive System Disorders, Immune System Diseases, and Skin and Musculoskeletal Diseases. AbbVie has also developed drugs for Respiratory Diseases, Nervous System Diseases, Eye Diseases, Hemic and Lymphatic Diseases, Infectious Diseases, Urogenital Diseases, Endocrinology and Metabolic Disease, Congenital Disorders, and Cardiovascular Diseases.
With its focus on addressing unmet medical needs and advancing novel therapies, AbbVie continues to contribute to the advancement of biomedicine and the improvement of patient outcomes.