Novartis releases positive Phase 3 trial data for its PCSK9-targeted siRNA therapy, inclisiran
On August 29, 2023, Novartis released new long-term data from its ORION-8 Phase 3 clinical trial. The data showed that its twice-yearly RNAi therapy, Leqvio (Inclisiran, Sodium Echociliran), in combination with statins, can continuously reduce Low-Density Lipoprotein Cholesterol (LDL-C) for over 6 years in patients with increased risk of Atherosclerotic Cardiovascular Disease (ASCVD), ASCVD or Heterozygous Familial Hypercholesterolemia (HeFH).
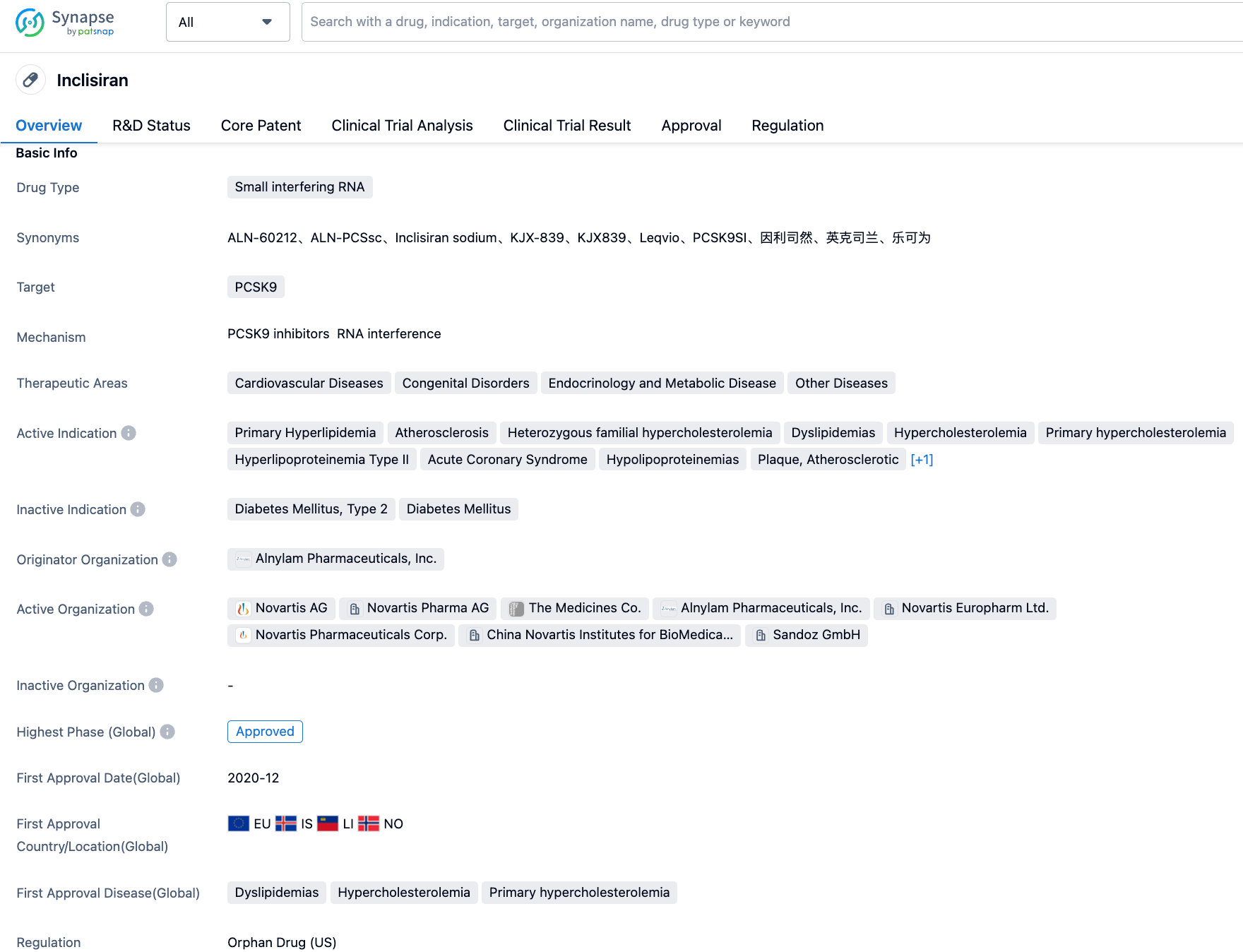
Inclisiran is a short-chain synthetic siRNA targeting PCSK9 developed by Novartis, consisting of two complementary ribonucleic acid chains, one of which acts as a guide and the other as a passenger. Upon binding with the multi-protein complex known as RISC (RNA-induced Silencing Complex), the guide chain of Inclisiran can hybridize with the complementary mRNA of PCSK9 and induce its degradation. Due to this unique mechanism of action, Inclisiran can reduce both intracellular and extracellular PCSK9 protein levels, resulting in a significant reduction of LDL-C concentration. Notably, the silencing complex remains active even after mRNA degradation, making the lipid-lowering effect of Inclisiran long-lasting. As the first-in-class small interfering RNA (siRNA) therapy for lowering Low-Density Lipoprotein Cholesterol (LDL-C), Inclisiran was approved in the European Union in December 2020 for the treatment of adult hypercholesterolemia and mixed dyslipidemia. Its innovative small interfering RNA mechanism allows patients to achieve lipid-lowering effects with just two subcutaneous injections per year. On August 22, 2022, Inclisiran was approved by the National Medical Products Administration (NMPA) of China as an adjunct to diet for the treatment of adult primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The results of the ORION-8 open-label extension trial showed that with two annual treatments of Leqvio in addition to statin therapy, patients' low-density lipoprotein cholesterol (LDL-C) can be continuously reduced after 6 years of treatment. 8 out of 10 cases reached the target LDL-C threshold, consistent with previously reported phase 3 data. Long-term safety data is consistent with previous results, affirming that Leqvio therapy has a confirmed and beneficial safety profile.
The key phase III, placebo-controlled, double-blind ORION (ORION-9, -10 and -11) clinical trial results showed that for patients who still cannot reach the LDL-C standard after the treatment with the maximum tolerated dose of statin drugs, Inclisiran can continuously reduce the level of LDL-C, with a reduction of up to 52%. Moreover, with an administration scheme of only two injections per year following initial treatment at the first and third months, Inclisiran is expected to resolve the dilemma of long-term patient adherence. In November 2022, Novartis published the results of the open-label extension part of the phase II ORION-1 trial (ORION-3). The results showed that the subjects' LDL-C level continued to decrease during the 4-year study period, where the LDL-C of patients treated with Leqvio was reduced by an average of 47.5% (95% Cl:-50.69, -44.27) from the baseline (day 1 of ORION-1) to day 210. In addition, about 80% of patients had an LDL-C level below 70mg/dL.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
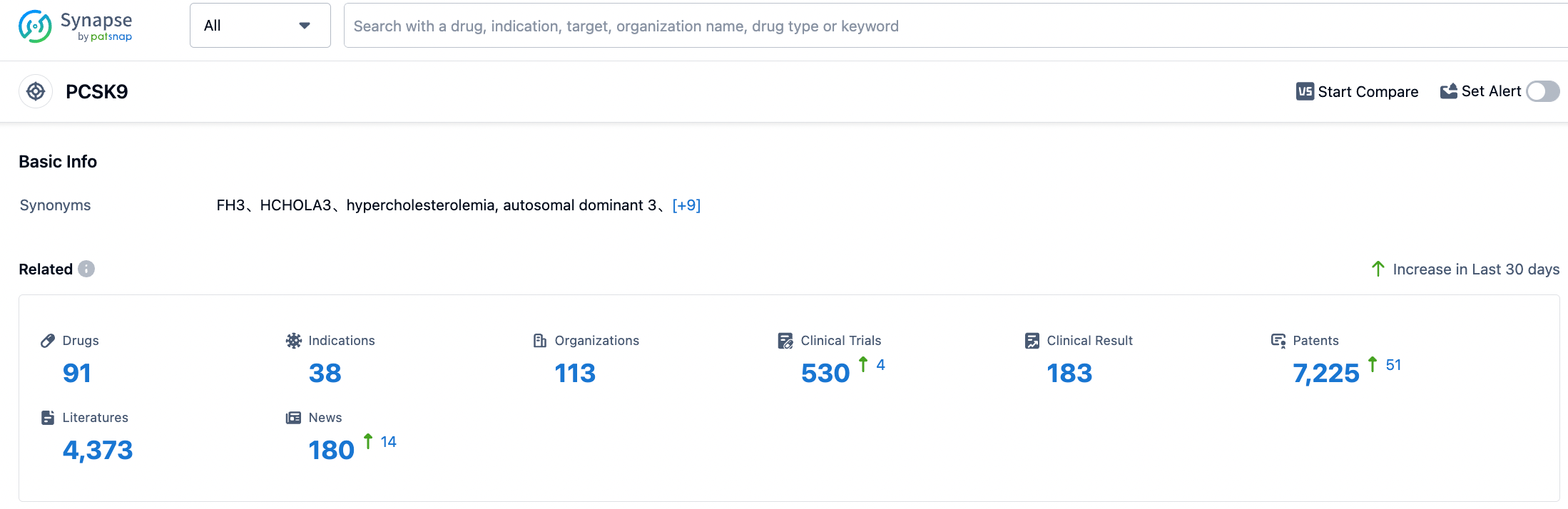
According to the information disclosed by the Synapse Database, as of August 30, 2023, there are a total of 91 investigational drugs targeted at PCSK9, encompassing 38 indications, developed by 114 institutions, involving 530 clinical trials, and as many as 7229 patents. The current competition in the PCSK9 inhibitor field is already quite intense. Cinda Bio's lipid-lowering drug, PCSK9 inhibitor Tolasizumab, has recently been approved for marketing. The marketing applications of Junshi Bio's Angorizumab, Hengrui Medicine's Ruikasizumab, and Kangfang Bio's Inusizumab are all under review. The red-sea market has begun to show signs. Only products with superior efficacy, higher safety, better compliance, and more affordable prices that have differentiated advantages can continuously grab market share and stand out. We look forward to the market performance of Inclisiran at home and abroad.