An Overview of Horizon Therapeutic’s Drug Pipeline | R&D Status
Horizon Therapeutics Plc is a pharmaceutical organization based in Dublin, Ireland. It was founded in 2008 and has since been actively involved in the field of biomedicine. The company has a diverse portfolio of drugs targeting various therapeutic areas. Horizon Therapeutics is a commercially-staged biotechnology company focused on developing drugs for the treatment of rare, autoimmune, and severe inflammatory diseases to meet pressing needs in these disease areas. Horizon's R&D goal is to apply scientific expertise to bring clinically meaningful therapies to patients and transform lives.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Horizon Therapeutic.
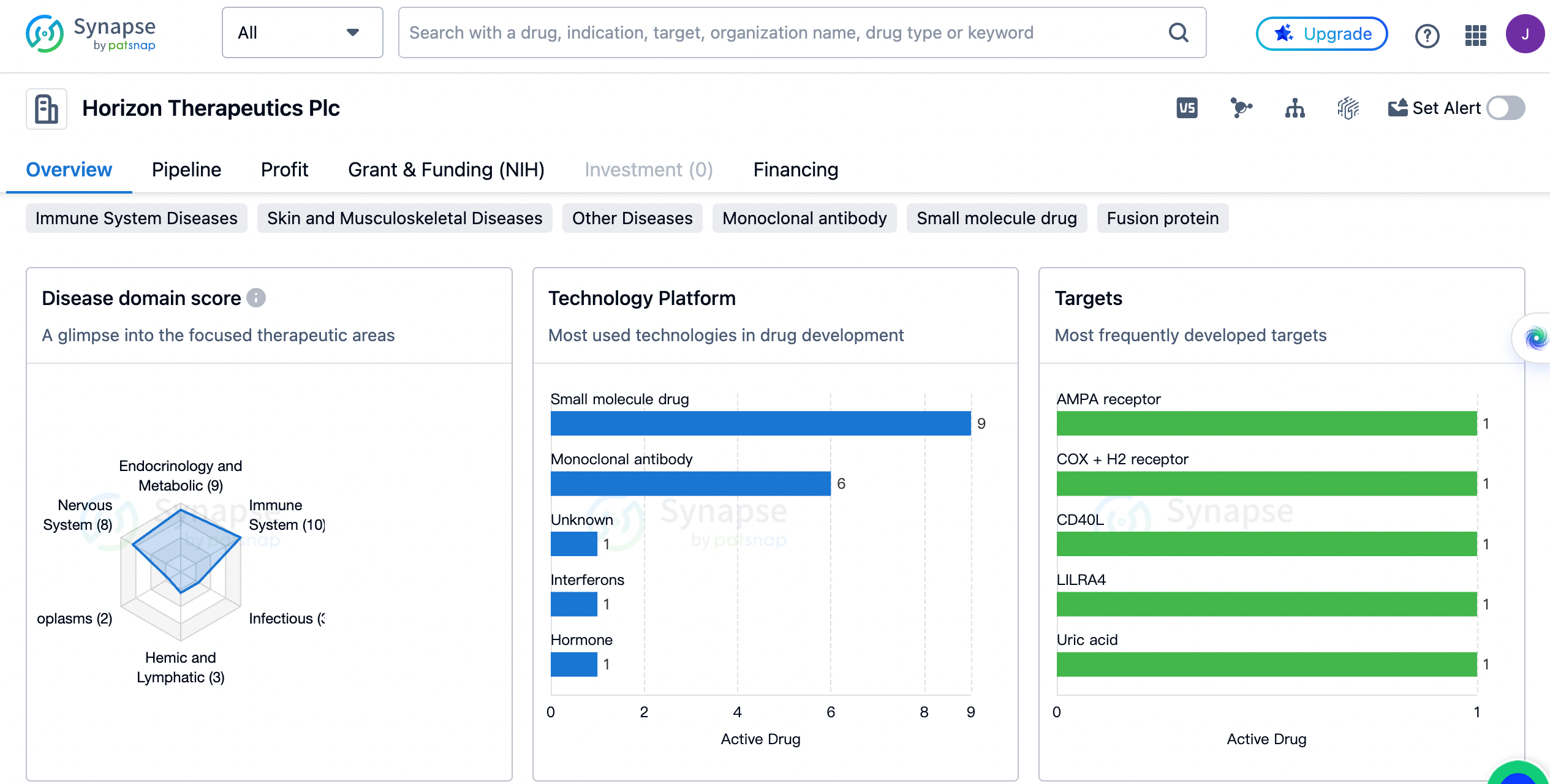
The distribution of therapeutic areas
Horizon Therapeutics Plc has developed drugs for a wide range of therapeutic areas. The highest drug count is in the field of Skin and Musculoskeletal Diseases, with 12 drugs. This indicates that the company has a strong focus on developing treatments for conditions related to the skin and musculoskeletal system. Other Diseases and Immune System Diseases follow closely behind with 11 and 10 drugs respectively. This suggests that Horizon Therapeutics Plc is also actively involved in developing treatments for a variety of other diseases and immune system disorders.
Endocrinology and Metabolic Disease, Nervous System Diseases, and Congenital Disorders have 9, 8, and 8 drugs respectively. These therapeutic areas also receive significant attention from the company, indicating a commitment to addressing conditions related to the endocrine system, nervous system, and congenital disorders.
Eye Diseases, Respiratory Diseases, Hemic and Lymphatic Diseases, and Infectious Diseases have a lower drug count, ranging from 5 to 3. This suggests that Horizon Therapeutics Plc has a relatively smaller focus on these therapeutic areas compared to others. Mouth and Tooth Diseases, Digestive System Disorders, Neoplasms, Urogenital Diseases, and Cardiovascular Diseases have even lower drug counts.
The most frequently developed targets by Horizon Therapeutics
Each target is associated with a drug count of 1, indicating that the company has developed drugs targeting a diverse range of molecular targets. Some of the targets mentioned include AMPA receptor, COX + H2 receptor, CD40L, LILRA4, Uric acid, IGF-1R, INSR, LPAR1, GR, CD19, COX, HDAC, IFNγR1, BChE + amylin, Transglutaminases, and CD32A. This demonstrates the company's commitment to exploring various molecular targets in the development of their drugs.
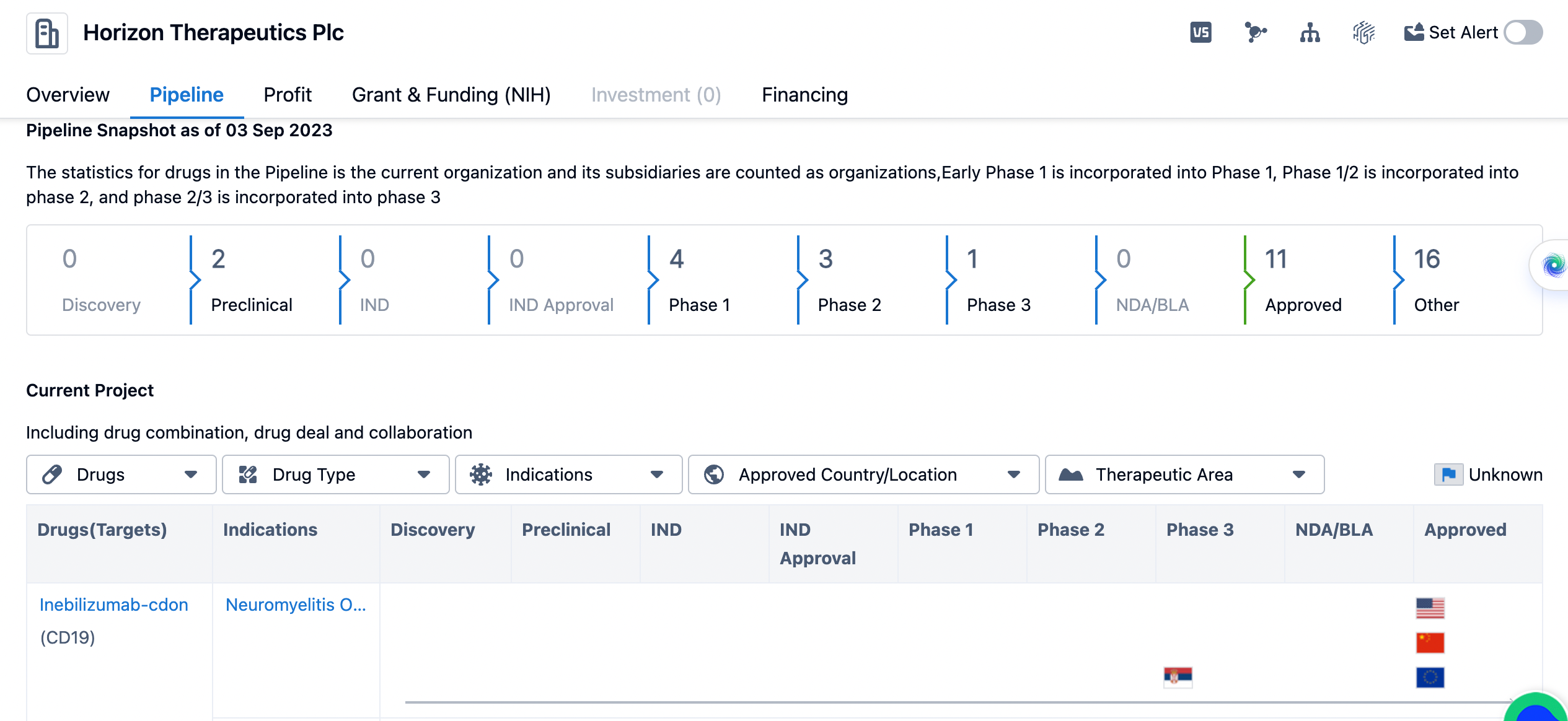
The pipeline of Horizon Therapeutics
The pipeline is categorized into different phases of drug development. The company has 2 drugs in the preclinical phase, indicating that they are in the early stages of development. In terms of clinical development, the company has 4 drugs in Phase 1, 3 drugs in Phase 2, and 1 drug in Phase 3. This indicates that Horizon Therapeutics Plc has a significant number of drugs in the clinical trial phase, with some nearing the final stages of development. The pipeline also shows that Horizon Therapeutics Plc has 11 drugs that have been approved. This indicates that the company has successfully brought several drugs to market and received regulatory approval for their use. Additionally, there are 16 drugs listed under "Other," which could include drugs in various stages of development or those that do not fit into the traditional phases mentioned in the pipeline.
In summary, Horizon Therapeutics Plc is a pharmaceutical organization based in Dublin, Ireland, specializing in the field of biomedicine. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a strong focus on Skin and Musculoskeletal Diseases, Other Diseases, and Immune System Diseases. Horizon Therapeutics Plc has developed drugs targeting a wide range of molecular targets, indicating a commitment to exploring different avenues in drug development. The company has a significant number of drugs in the clinical trial phase, with some nearing the final stages of development. Additionally, Horizon Therapeutics Plc has successfully brought several drugs to market and received regulatory approval for their use.