EMA has applied for marketing authorization of ARCT-154 vaccine for the prevention of COVID-19 through Arcturus Therapeutics and CSL
The worldwide clinical-stage messenger RNA medicines corporation, Arcturus Therapeutics Holdings Inc., with a focus on creating vaccines for infectious diseases and exploring potential treatments for rare liver and respiratory ailments, along with CSL and its division specializing in vaccines, have revealed today that EMA has officially confirmed the marketing authorization application for ARCT-154. This advanced mRNA vaccine is designed to actively immunize against COVID-19 contracted from SARS-CoV-2 virus in individuals who are 18 years and older.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The EMA submission is based on successful Phase 3 clinical results of ARCT-154 against the ancestral D614G variant as a primary series and booster. In evaluating the six-month data from the key stage 3 trial, the vaccine hit the main effectiveness objectives, with ARCT-154 achieving 56.6% efficacy in averting symptomatic COVID-19 overall, and a 95.3% success rate in preventing severe COVID-19 cases, including COVID-19-linked deaths.
"EMA's approval of ARCT-154's commercialization submission marks yet another significant stride in the evolution of this pioneering mRNA vaccine technology," stated Joseph Payne, the President and CEO of Arcturus Therapeutics. "ARCT-154 is anticipated to be quickly updated in response to emerging variants of concern and could potentially serve as a longer-lasting shield against this widespread, mutating COVID virus."
"This vital regulatory progress moves CSL a step nearer to delivering groundbreaking mRNA vaccines to Europe," commented Emmanuelle Lecomte Brisset, who heads the Global Regulatory Affairs at CSL. "We eagerly await the opportunity to collaborate with regulatory bodies to further enhance our defense of public health."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
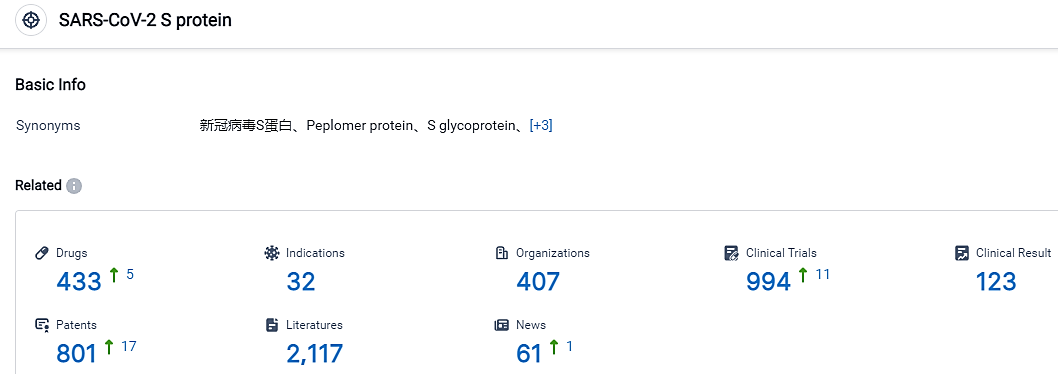 According to the data provided by the Synapse Database, As of September 7, 2023, there are 433 investigational drugs for the SARS-CoV-2 S protein, including 32 applicable indications, 407 R&D institutions involved, with related clinical trials reaching 994, and as many as 801 patents.
According to the data provided by the Synapse Database, As of September 7, 2023, there are 433 investigational drugs for the SARS-CoV-2 S protein, including 32 applicable indications, 407 R&D institutions involved, with related clinical trials reaching 994, and as many as 801 patents.
Arcturus Therapeutics Holdings, Inc. has developed an RNA vaccine known as ARCT-154. It is designed to target the S protein of SARS-CoV-2 and its primary objective is to prevent and treat COVID-19. ARCT-154, with its progressed level of research and potential benefits, could be instrumental in the battle against the persisting pandemic.





