An Overview of ImmunoGen’s Drug Pipeline
ImmunoGen, Inc. is a biopharmaceutical company that was founded in 1981 and is based in Massachusetts, United States. The company specializes in the development of targeted antibody-drug conjugates (ADCs) for the treatment of various diseases, particularly neoplasms and hematological disorders. Previously, ImmunoGen, Daiichi Sankyo and Seagen were considered to have the strongest overseas ADC technology platform. ImmunoGen's ADC platform has a long history and leading technology, and has built a four-in-one ADC tool library for target screening, antibody development, toxin library, and linker library. ImmunoGen's technology gave birth to Kadcyla, the all-time best-selling ADC.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of ImmunoGen.
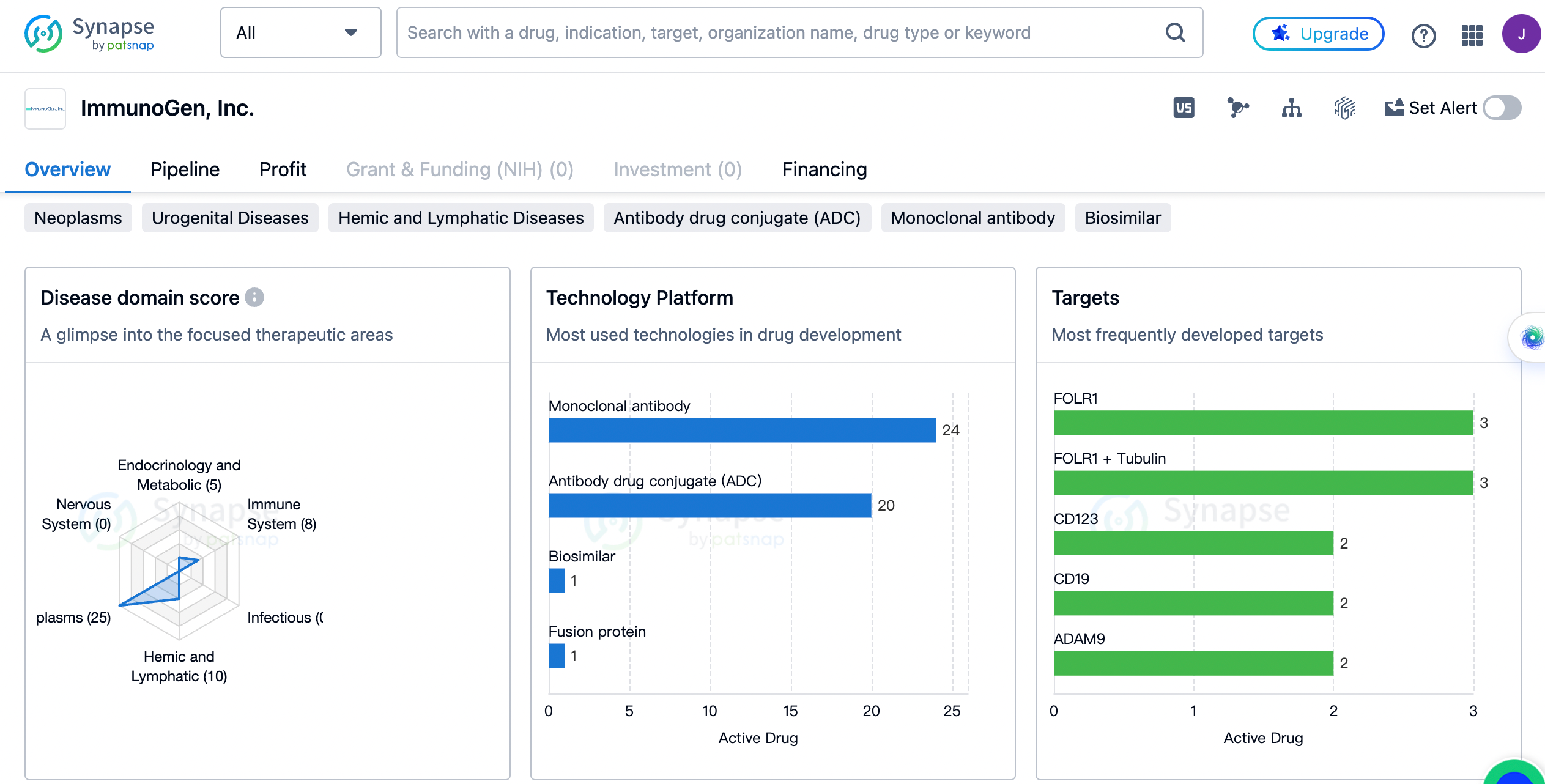
ImmunoGen has a diverse portfolio of drugs targeting different therapeutic areas. The highest number of drugs developed by the company are for the treatment of neoplasms, with a count of 25. This indicates that ImmunoGen has a strong focus on developing therapies for cancer. Hemic and lymphatic diseases are the second most targeted therapeutic area, with 10 drugs in development. Immune system diseases and skin and musculoskeletal diseases are also areas of interest for the company, with 8 and 6 drugs respectively. Other therapeutic areas targeted by ImmunoGen include digestive system disorders, endocrinology and metabolic diseases, urogenital diseases, respiratory diseases, congenital disorders, cardiovascular diseases, eye diseases, and other diseases.
The most frequently developed targets by ImmunoGen
FOLR1 and FOLR1 + Tubulin are the most targeted, with 3 drugs each. FOLR1 is a receptor that is overexpressed in many types of cancer cells, making it an attractive target for ADC therapy. CD123 and CD19 are also popular targets, with 2 drugs each. CD123 is a cell surface receptor that is highly expressed on leukemia cells, while CD19 is a protein found on the surface of B cells. Other targets include ADAM9, CD56 + Tubulin, Tubulin + c-Met, DNA + Guanylate cyclase, CD19 + Tubulin, CD56, ENG + Tubulin, IGF-1R, CD33, IL-12 + IL-23, CD37 + Tubulin, CD166 + Tubulin, and CD20 + IFNAR.
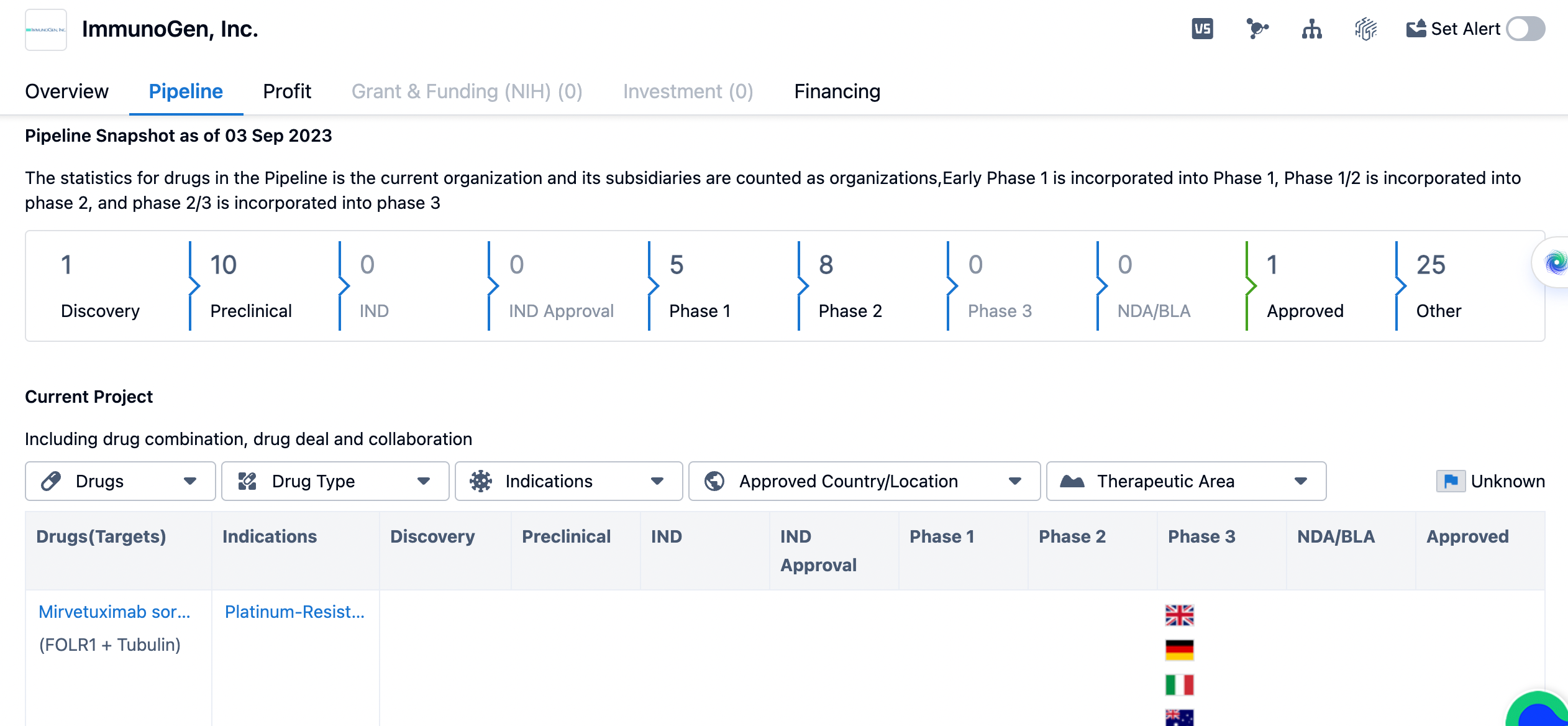
The pipeline of ImmunoGen
In terms of the pipeline, ImmunoGen has a number of drugs at different stages of development. The majority of drugs are in the preclinical stage, with a count of 10. This indicates that ImmunoGen is actively conducting research and development to identify potential drug candidates. The company has 5 drugs in Phase 1, which suggests that these drugs have shown promising results in preclinical studies and are now being tested in humans for the first time. Phase 2 is the next stage of development, and ImmunoGen has 8 drugs in this phase. Phase 2 trials involve a larger number of patients and aim to further evaluate the safety and efficacy of the drugs. ImmunoGen has 1 drug that has been approved. The remaining drugs are in various stages of the development process, including discovery, IND, IND approval, Phase 3, NDA/BLA, and other stages.
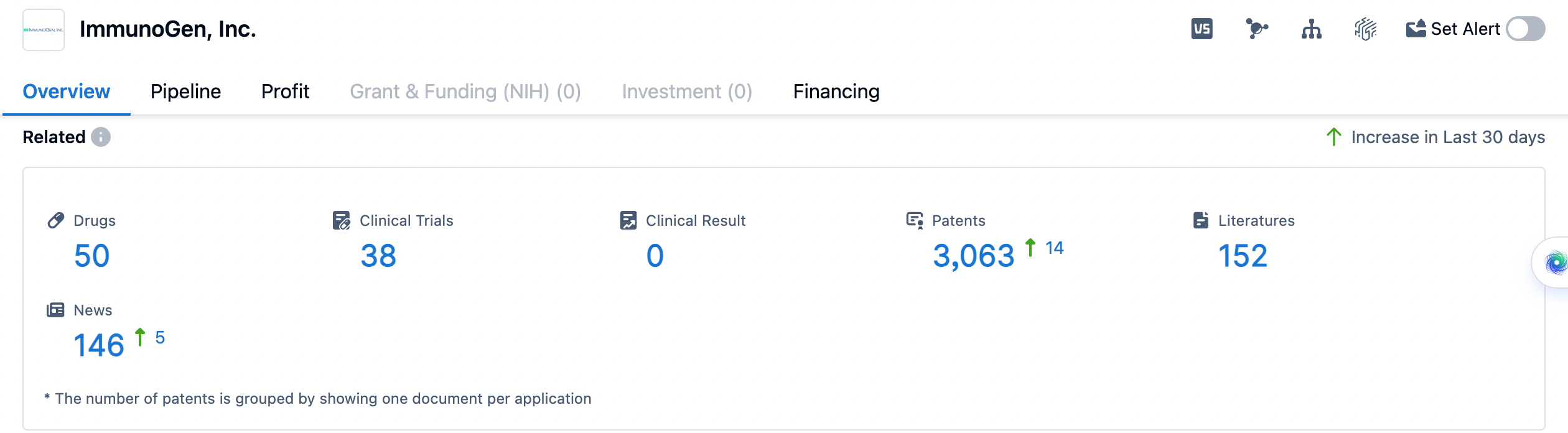
In summary, ImmunoGen, Inc. is a biopharmaceutical company that focuses on the development of targeted antibody-drug conjugates for the treatment of various diseases, particularly neoplasms and hematological disorders. The company has a diverse portfolio of drugs targeting different therapeutic areas, with a strong emphasis on cancer. ImmunoGen targets a range of proteins and receptors, with FOLR1 and CD123 being the most frequently targeted. The company has a number of drugs in its pipeline at different stages of development, with a significant focus on preclinical and Phase 2 trials. ImmunoGen has successfully brought one drug to market and continues to actively research and develop new therapies.