An Overview of Ionis Pharmaceutical’s Drug Pipeline
Ionis Pharmaceuticals, Inc. is a biopharmaceutical company that was founded in 1989 and is headquartered in California, United States. The company has been actively involved in the development of drugs in various therapeutic areas.
As a leader in RNA-targeted drug discovery and development, Ionis has created an efficient, widely applicable drug discovery platform called Antisense Technology that can treat diseases for which no other effective therapies have been discovered. Ionis' drug discovery platform provides a viable promise and a springboard to hope for patients with unmet needs, creating Spinraza, the first approved drug for the treatment of spinal muscular atrophy in children and adults, and Tegsedi, the world's first approved RNA-targeted therapy for adult polyneuropathy with hereditary transthyroxine amyloidosis. Ionis aims to make more than 40 novel drugs available to all patients who have not yet been exposed to the potential for the treatment of a range of diseases, including neurological, cardiovatic, infectious and pulmonary diseases. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Ionis Pharmaceuticals, Inc.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Ionis Pharmaceutical.
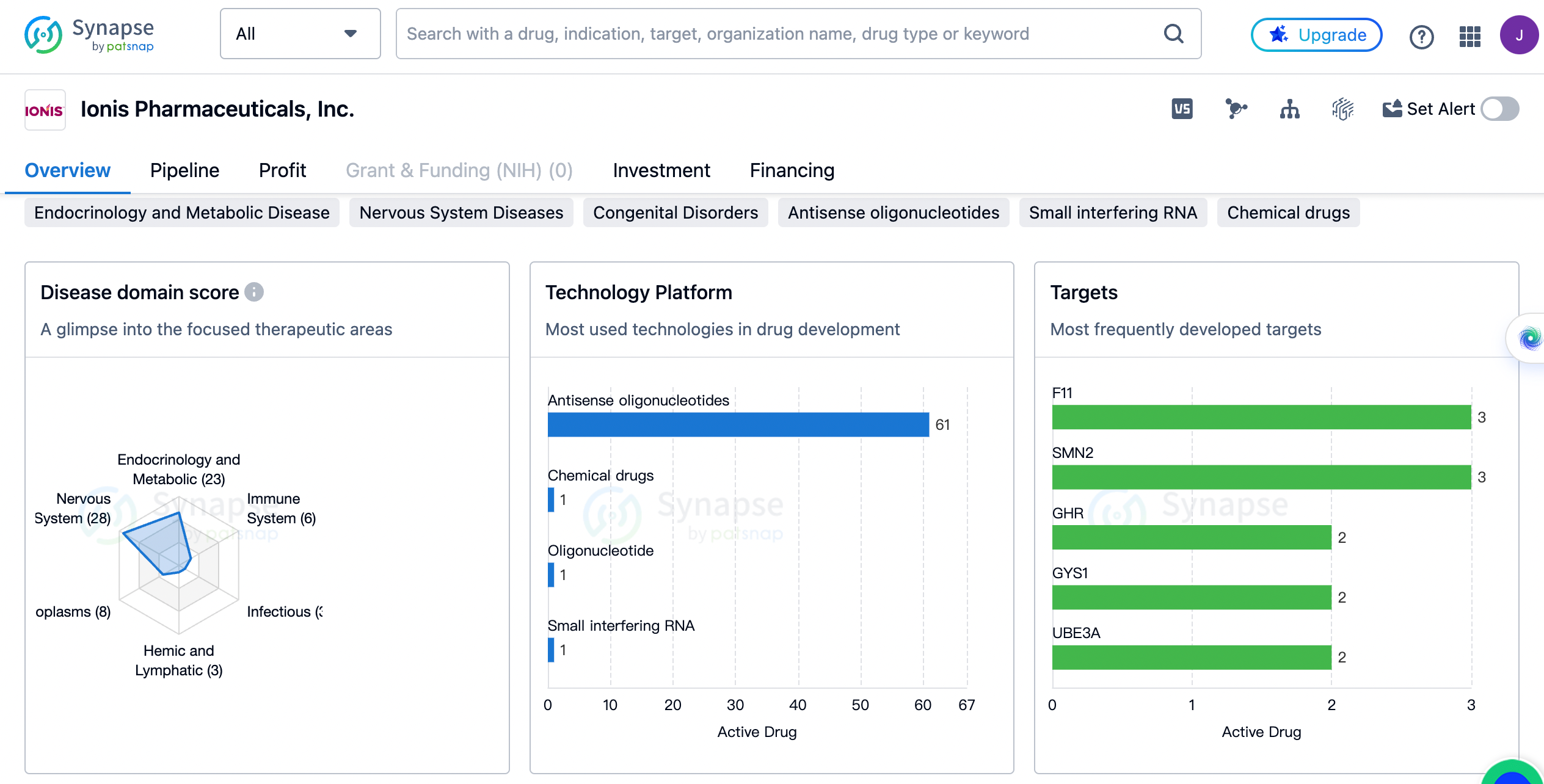
Ionis Pharmaceuticals has developed drugs in a wide range of therapeutic areas. The highest number of drugs, 28 in total, are focused on Nervous System Diseases. This indicates that the company has a strong emphasis on developing treatments for neurological disorders. Following closely behind are Endocrinology and Metabolic Disease with 23 drugs, and Congenital Disorders with 18 drugs. These therapeutic areas also demonstrate the company's commitment to addressing diseases related to the endocrine system and genetic disorders.
Cardiovascular Diseases, Digestive System Disorders, and Neoplasms are the next most targeted therapeutic areas, with 13, 11, and 8 drugs respectively. This suggests that Ionis Pharmaceuticals, Inc. recognizes the significant burden of these diseases and is actively working towards developing innovative treatments. The company has also developed drugs for Skin and Musculoskeletal Diseases, Immune System Diseases, Urogenital Diseases, Hemic and Lymphatic Diseases, Respiratory Diseases, Infectious Diseases, and Eye Diseases, although to a lesser extent.
The most frequently developed targets by Ionis Pharmaceuticals
The targets F11 and SMN2 have the highest number of drugs, with 3 each. This indicates that the company has dedicated significant resources to developing treatments for diseases associated with these targets. GHR, GYS1, UBE3A, AGT, TTR, and APOC3 are the next most frequently developed targets, with 2 drugs each. These targets represent a diverse range of diseases, including growth hormone-related disorders, glycogen storage diseases, and genetic disorders.
An overview of the pipeline of Ionis Pharmaceuticals
The majority of drugs are in the Preclinical phase, with 18 in total. This suggests that the company is actively conducting research and development activities to identify potential drug candidates. Following the Preclinical phase, there are 7 drugs in Phase 1, 25 drugs in Phase 2, and 8 drugs in Phase 3. These numbers indicate that Ionis Pharmaceuticals, Inc. has a robust pipeline with drugs at various stages of development.
In terms of regulatory milestones, there are 2 drugs in the NDA/BLA stage, indicating that they have submitted New Drug Applications or Biologics License Applications to regulatory authorities. Additionally, 3 drugs have received approval, demonstrating the successful commercialization of these products. It is worth noting that there are 58 drugs categorized as "Other" in the pipeline. This category may include drugs in early discovery stages, as well as those in late-stage development but not yet categorized under a specific phase.
In summary, Ionis Pharmaceuticals, Inc. is a biopharmaceutical company that has been actively involved in the development of drugs across various therapeutic areas. The company has a strong focus on Nervous System Diseases, Endocrinology and Metabolic Disease, and Congenital Disorders. They have also targeted diseases in the areas of Cardiovascular Diseases, Digestive System Disorders, Neoplasms, and others. Ionis Pharmaceuticals, Inc. has developed drugs targeting a diverse range of targets, with F11 and SMN2 being the most frequently developed targets. The company has a robust pipeline with drugs at different stages of development, from Preclinical to Approved. This indicates their commitment to advancing innovative treatments for a wide range of diseases.