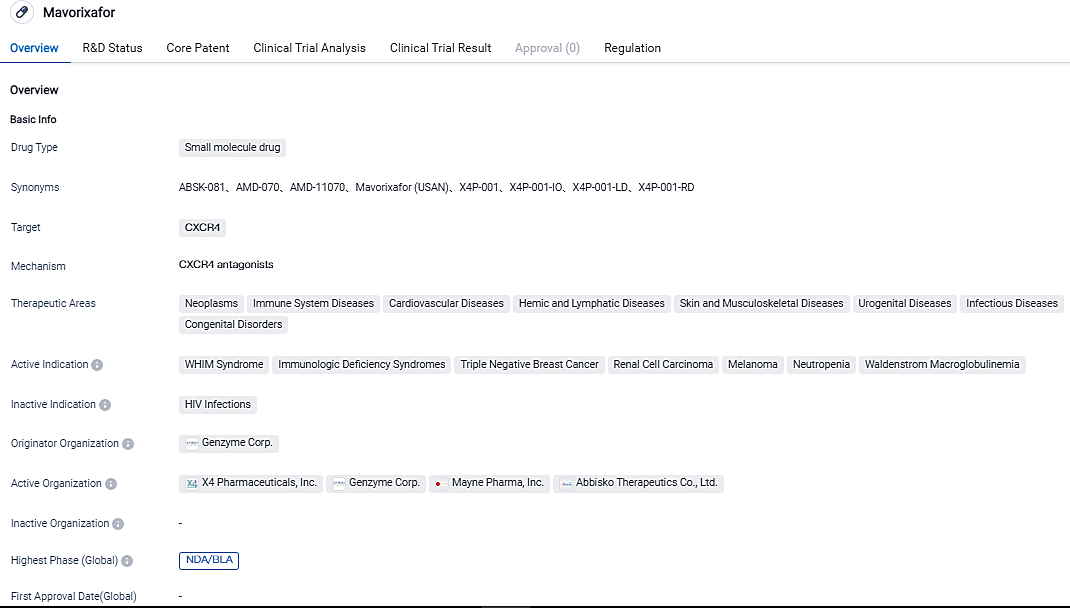
X4 Pharmaceuticals has submitted a New Drug Application to the FDA for Mavorixafor WHIM Syndrome
X4 Pharmaceuticals has submitted a New Drug Application (NDA) to the FDA for the approval of once-daily oral mavorixafor in the treatment of WHIM syndrome, a rare primary immunodeficiency, in individuals aged 12 and above. X4 Pharmaceuticals is dedicated to enhancing the quality of life for individuals with rare immune system disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The submission of our NDA marks a significant milestone in the progression of X4, aimed at ameliorating the health care of those affected by uncommon immunodeficiency disorders,” stated Paula Ragan, Ph.D., the CEO and President of X4 Pharmaceuticals. “This progress brings us nearer to the potential unveiling of the inaugural product in the U.S. market for those suffering from WHIM syndrome. Concurrently, we are making strides in our clinical program that evaluates the effect of mavorixafor on those with chronic neutropenic disorders.”
The FDA usually takes up to 60 days to investigate whether an NDA meets the complete criteria before approving it. X4 has filed a request for prioritized assessment of the application and if accepted, this would set a six-month deadline for the FDA to review the application from the date of acceptance.
The NDA's submission relies on the findings from the worldwide, pivotal 4WHIM Phase 3 clinical trial that tested the oral, daily dosage of mavorixafor on people diagnosed with WHIM syndrome. The 4WHIM trial successfully achieved its primary and key secondary objectives and was generally well received without reporting any serious side effects related to treatment or stopping due to safety issues.
WHIM syndrome is a sporadic, hereditary, immunodeficiency disorder caused by the reduced mobilization and migration of white blood cells from bone marrow owing to the excessive signaling of the CXCR4/CXCL12 pathway. Those struggling with WHIM syndrome typically have extremely low neutrophil and lymphocyte levels in their blood, often leading to repeated infections, an increased risk of lung disorders, persistent warts caused by an underlying HPV infection, scarce immunoglobulin production, and higher risk to develop certain types of cancer.
The 4WHIM Phase 3 clinical trial was a global, randomized, double-blind, placebo-controlled, multi-center study, set up to ascertain the efficacy and safety of oral, once-daily dosages of mavorixafor on people with genetically proven WHIM syndrome. A total of 31 participants aged 12 and above were enrolled in the trial, who received either mavorixafor (n=14) or placebo (n=17) orally once daily for an entire year.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 7, 2023, there are 77 investigational drugs for the CXCR4 target, including 114 applicable indications,92 R&D institutions involved, with related clinical trials reaching 294,and as many as 14221 patents.
Mavorixafor, a small-molecule antagonist of CXCR4 under investigation, is being formulated as a daily oral treatment for WHIM syndrome. For its potential use against WHIM syndrome, mavorixafor has received Breakthrough Therapy Designation, Fast Track Designation, and Rare Pediatric Designation in the U.S, as well as Orphan Drug Status in both the U.S and European.