Analysis on the Clinical Research Progress of Prostaglandin Synthases Inhibitors
Prostaglandin synthases are enzymes that play a crucial role in the human body by catalyzing the synthesis of prostaglandins. Prostaglandins are lipid compounds that act as local hormones, exerting diverse physiological effects. These synthases are responsible for converting arachidonic acid, a fatty acid, into prostaglandins, which are involved in various biological processes such as inflammation, blood clotting, and regulation of blood pressure. Prostaglandin synthases are essential for maintaining homeostasis and are potential targets for pharmaceutical interventions in conditions like pain, inflammation, and cardiovascular diseases. Understanding their role and regulation is vital for developing therapeutic strategies to modulate prostaglandin levels and alleviate associated health issues.
Prostaglandin synthases Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 101 Prostaglandin synthases drugs worldwide, from 103 organizations, covering 125 indications, and conducting 108 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target Prostaglandin synthases reveals a competitive landscape with multiple companies making progress in the research and development of drugs. Kissei Pharmaceutical Co., Ltd., Wuhan Hengxinyuan Pharmaceutical Co Ltd, Sun Pharmaceutical Industries Ltd., and other companies have achieved approvals for drugs targeting Prostaglandin synthases.
The indications for these drugs cover a wide range of conditions, including Asthma, Thromboembolism, Hypertension, and Dermatitis. The drug types progressing rapidly include Small molecule drugs, Small molecule-drug conjugates, and Unknown drugs. The development of biosimilars indicates intense competition in the market.
Various countries, including Japan, China, and the European Union, are actively involved in the development of drugs targeting Prostaglandin synthases. Further analysis is required to understand the specific progress made by each country. Overall, the target Prostaglandin synthases present opportunities for growth and development in the pharmaceutical industry.
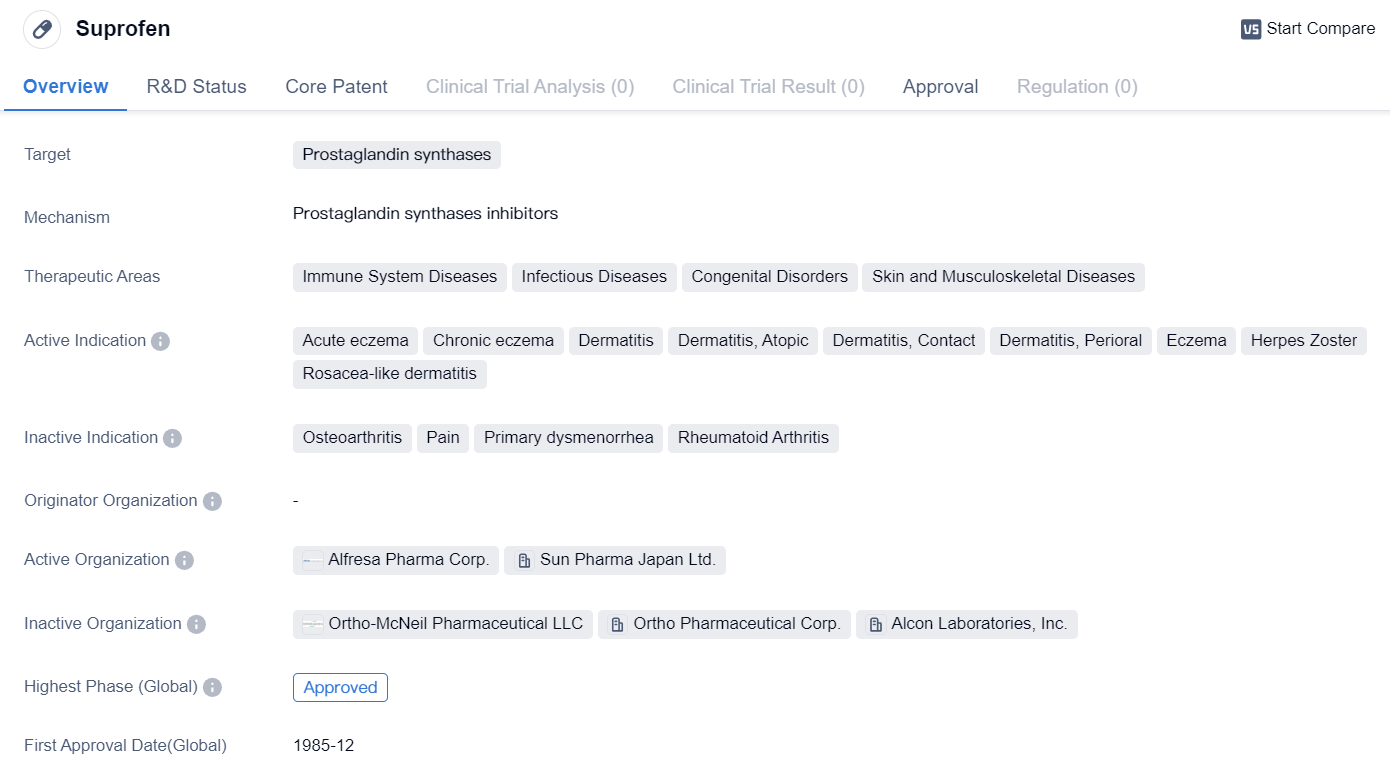
Approved Prostaglandin Synthases Inhibitors for Market Launch: Suprofen
Suprofen is a small molecule drug that belongs to the class of nonsteroidal anti-inflammatory drugs (NSAIDs). It primarily targets prostaglandin synthases, which are enzymes involved in the production of prostaglandins, lipid compounds that play a role in inflammation and pain.
This drug has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, congenital disorders, and skin and musculoskeletal diseases. Some specific indications for Suprofen include acute eczema, chronic eczema, dermatitis (including atopic, contact, and perioral dermatitis), eczema, herpes zoster, and rosacea-like dermatitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Suprofen received its first approval in the United States in December 1985. It is important to note that this information pertains to the highest phase of development for Suprofen, which is approved. This suggests that the drug has successfully completed clinical trials and has been deemed safe and effective for use in patients.
As a small molecule drug, Suprofen is likely administered orally or topically, depending on the specific indication and formulation. It is expected to exert its therapeutic effects by inhibiting prostaglandin synthesis, thereby reducing inflammation and alleviating associated symptoms.
Given the first approval date in 1985, Suprofen has been available for several decades, indicating its established presence in the pharmaceutical market. However, it is worth noting that the information provided does not specify the current status of Suprofen, such as its availability in different countries or any subsequent approvals or changes in its therapeutic indications.
In summary, Suprofen is a small molecule drug that targets prostaglandin synthases. It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, congenital disorders, and skin and musculoskeletal diseases. The drug is indicated for conditions such as eczema, dermatitis, herpes zoster, and rosacea-like dermatitis. Suprofen received its first approval in the United States in December 1985 and is considered an established drug in the pharmaceutical market.