Analysis on the Research Progress of Chk1 inhibitor
Chk1, also known as checkpoint kinase 1, is a crucial protein kinase involved in the regulation of cell cycle progression and DNA damage response in the human body.
Chk1 is a serine/threonine (Ser/Thr) kinase encoded by the tumor suppressor gene Chk1, broadly existing in mammalian cells. In recent years, numerous domestic and international studies have indicated that Chk1 and its inhibitors can regulate the formation and development of tumors. Chk1 controls cell proliferation, being the key gene leading to an S/G1 and G2/M phase arrest after DNA damage, thus ensuring DNA repair and having vital surveillance function. Overexpression of Chk1 is observed in a variety of tumor tissues; it can participate in the onset and progression of certain tumors through specific molecular signaling pathways, and its aberrant expression levels are closely related to the therapeutic effects and prognosis of patients with hematological tumors. High expression of Chk1 is of great significance in the infiltration and metastasis of hematological tumor cells, and the appearance of Chk1 inhibitors provides a new direction for the treatment of malignant blood tumors.
It plays a vital role in ensuring the integrity of the genome by monitoring DNA replication and repair processes. Chk1 acts as a checkpoint mediator, halting cell cycle progression in response to DNA damage or replication stress, allowing time for repair mechanisms to be activated. Dysregulation of Chk1 has been implicated in various diseases, including cancer, making it an attractive target for pharmaceutical interventions aimed at enhancing the efficacy of DNA-damaging therapies or developing novel anticancer treatments.
So far, over 10 types of CHK1 inhibitors have entered clinical research, but no CHK1 inhibitor has been approved for marketing. Most CHK1 inhibitors have ceased development due to issues of efficacy and safety, and most of these compounds are administered through injections. Of the 4 types of CHK1 inhibitors that are currently still under clinical research, besides LY2606368, GDC0575, LY2880070, and CCT245737 can all be administered orally, greatly improving patient compliance. Therefore, the development of highly active, highly selective, and orally administrable new CHK1 inhibitors is an important direction for research in this field.
Studies have found that the absence of FAM122A can promote tumor cell resistance to CHK1 and ATR inhibitors.
Further research showed that CHK1 can phosphorylate the S37 site of FAM122A, which makes it move out of the nucleus and bind with 14-3-3, inhibiting its binding with the PP2A-B55α phosphatase complex, thereby improving the stability of WEE1 protein. Similarly, the absence of FAM122A will also increase the stability of WEE1 protein, activate the G2/M checkpoint, and prevent cells with DNA damage from entering mitosis, achieving resistance.
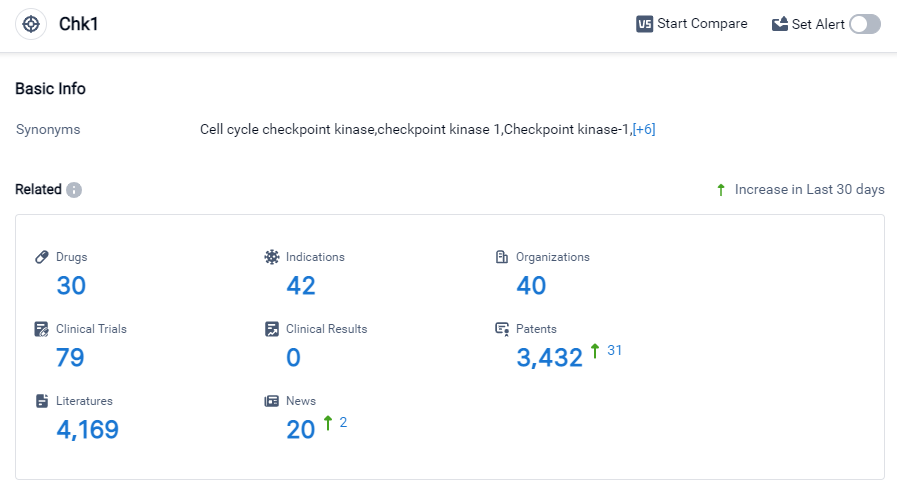
Chk1 Competitive Landscape
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 30 Chk1 drugs worldwide, from 40 organizations, covering 42 indications, and conducting 79 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the provided data, the current competitive landscape of target Chk1 shows that Eli Lilly & Co., Pfizer Inc., GSK Plc, and Baylor University are the companies with the highest development stages. Ovarian Cancer and Uterine Cervical Cancer are the indications with the most drugs in phase 2 development. Small molecule drugs are progressing rapidly under the current target, with a significant number of drugs in both phase 2 and preclinical stages. The United States, United Kingdom, and European Union are the countries/locations with the highest development stages, while China also shows progress in phase 1 development. Overall, the target Chk1 presents opportunities for further research and development in various indications and drug types, with a focus on small molecule drugs.