The FDA grants Merck accelerated consideration for their new BLA for the Activin Signaling Inhibitor, Sotatercept
Merck, identified as MSD in countries beyond the United States and Canada, has declared that a fresh BLA for sotatercept has been approved for accelerated assessment by the U.S. FDA. This new activin signaling inhibitor from Merck is designed for treating adult patients suffering from pulmonary arterial hypertension. The FDA has determined a Prescription Drug User Fee Act date, alternatively called target action, which is due on March 26, 2024.
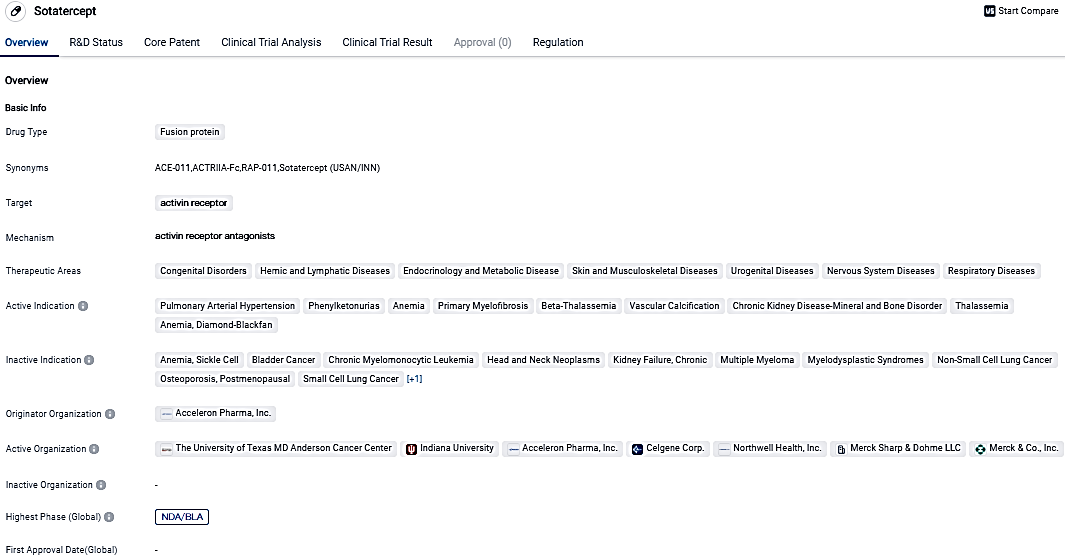
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
PAH is marked by the constriction of pulmonary blood vessels, which puts extreme pressure on the heart. The submission for sotatercept utilizes the results from the Phase 3 STELLAR test. It showed that when combined with baseline treatment, sotatercept made a significant and valuable difference in 6-minute walk distance and eight out of nine secondary assessments.
"Even with medical progress in PAH over the past twenty years, it's crucial that patient outcomes continue to improve," stated Dr. Joerg Koglin, senior vice president of Merck Research Laboratories' global clinical development.
"This application's acceptance by the FDA is an exciting step towards bringing this groundbreaking therapeutic option, an activin signaling inhibitor, to patients in need. Based on the considerable improvements in both primary and secondary indicators in the Phase 3 STELLAR study, we think sotatercept can significantly impact PAH patients' treatment," added Dr. Joerg Koglin.
Sotatercept, a potential pioneer in the class of activin signaling inhibitor biologics, is being researched as a treatment for PAH. An infrequent illness, PAH, results from the overgrowth of cells in the lung's arterial walls, leading to abnormal narrowing and tightening. Preliminary studies suggest that sotatercept can control vascular cell growth, undoing vascular and right ventricle restructuring.
Beyond STELLAR, the sotatercept clinical development plan incorporates numerous Phase 2 and 3 trials across a wide range of patients. These trials include adult PAH patients at medium or high risk of disease worsening or death and those with pulmonary hypertension due to left heart disease.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 8, 2023, there are 5 investigational drugs for the activin receptor, including 27 indications,12 R&D institutions involved, with related clinical trials reaching 35,and as many as 2127 patents.
Sotatercept has been granted Breakthrough Therapy Designation and Orphan Drug designation by the U.S. FDA, as well as Priority Medicines designation and Orphan Drug designation by the European Medicines Agency for the treatment of PAH. Merck acquired exclusive rights to sotatercept in the pulmonary hypertension field through the acquisition of Acceleron Pharma Inc. Sotatercept is the subject of a licensing agreement with Bristol Myers Squibb.